Antioxidative potential of two chemically characterized Ocimum (Tulsi) species extracts
DOI:
https://doi.org/10.15419/bmrat.v4i9.366Keywords:
Flavonoids, HPLC profiling, Ocimum kilimandscharicum, Ocimum sanctum, Phenolics, Rosmarinic acidAbstract
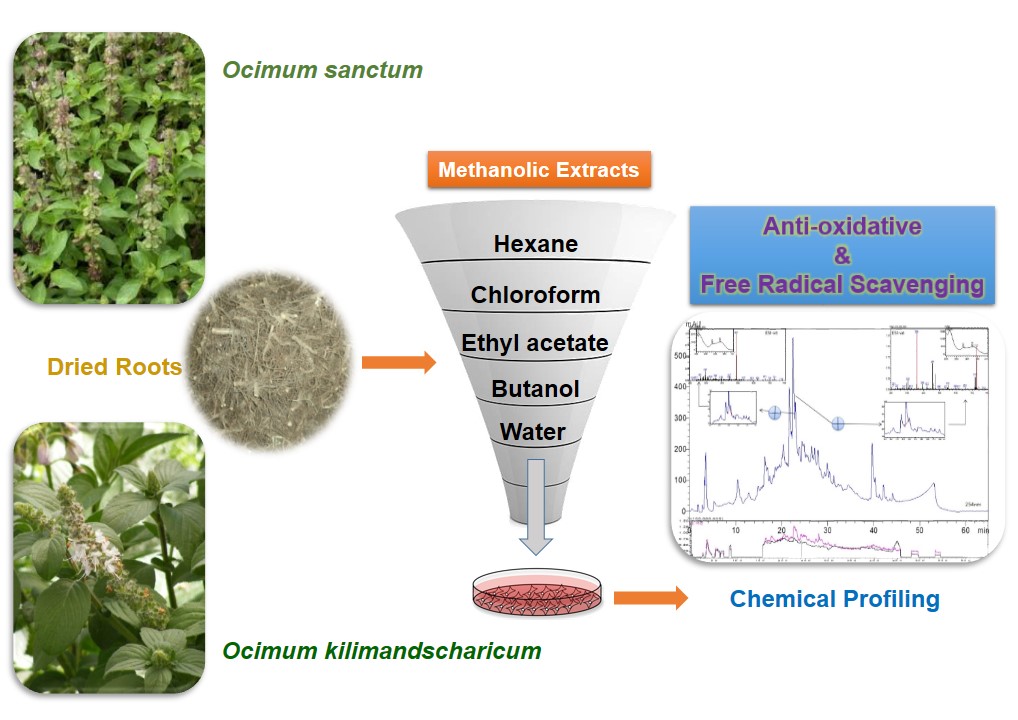
Background: Ocimum sanctum and Ocimum kilimandscharicum are cultivated in Indian subcontinent both for the religious and medicinal properties. Traditionally, the leaves have been reported for their enormous therapeutic potentials but the roots which are otherwise considered as a waste part have not been explored for their pharmacological activity.
Methods: Total phenolic content, free radical scavenging and ferric reducing antioxidant potential of various extracts from Ocimum sanctum and Ocimum kilimandscharicum were assessed and compared. In vitro antioxidant activity was estimated spectrophotometrically and the most potent ethyl acetate extract was chemically characterized by developing the chemical fingerprint and quantifying the probable constituents with the help of HPLC/LC-MS techniques.
Results: The ethyl acetate extract of both the species exhibited significant free radical scavenging potential and also reduced the ferric ions. It was observed that ethyl acetate extract have superior ferric reducing potential than other tested extracts, which were evidenced by high ferrous sulphate equivalent value of 77.05 ± 1.54 and 80.98 ± 0.80 at 100 µg/ml for O. sanctum and O. kilimandscharium respectively. The ferric reducing capacity of ethyl acetate extract for both the species was also evidenced by an elevated optical density of 1.64 ± 0.12 and 2.14 ± 0.08. Ocimum sanctum exhibited better antioxidant capacity (11.31 ± 0.20 AScE) as compared to Ocimum kilimandscharium (9.08 ± 0.27 AScE). The total phenolic and flavonoid content were estimated by spectrophotometric method and tentatively characterized by HPLC/LC-MS profiling which revealed the presence of rosmarinic acid, caffeic acid along with its derivatives such as caffeoyl-dihydroxyphenyllactoyl-tartaric acid.
Conclusion: The ethyl acetate extract of both the species being rich in phenolic and flavonoid contents exhibited potent antioxidant activity. The presence of flavonoid in ethyl acetate extracts further co-relates the antioxidative properties of roots extracts. To the best of our knowledge and understanding this is the first report of comparative chemical profiling by RP-HPLC/LC-MS and antioxidant potential of roots of two Ocimum species.
References
<li class="show">Ahmad, A., Singh, D. K., Fatima, K., Tandon, S., Luqman, S., (2014). New constituents from the roots of Oenothera biennis and their free radical scavenging and ferric reducing activity. Industrial Crops and Products 58, 125–132. <a href="https://doi.org/10.1016/j.indcrop.2014.04.008">https://doi.org/10.1016/j.indcrop.2014.04.008</a></li>
<li class="show">Ahmad, M. Z., Ali, M., Mir, S. R., (2012). Anti-diabetic activity of Ocimum sanctum L. roots and isolation of new phytoconstituents using two-dimensional nuclear magnetic resonance spectroscopy. Journal of Pharmacognosy and Phytotherapy 4, 75–85. <a href="https://doi.org/10.5897/JPP12.008">https://doi.org/10.5897/JPP12.008</a></li>
<li class="show">Akinmoladun, A. C., Ibukun, E. O., Afor, E., Obuotor, E. M., Farombi, E. O., (2007). Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum. Scientific Research and Essays 2, 163–166.</li>
<li class="show">Balaji, R., Prakash, G., Suganya, P. D., Aravinthan, K. M., (2011). Antioxidant activity of methanol extract of Ocimum tenuiflorum (dried leaf and stem). Int J Pharm Sci Rev Res 3, 20–7.</li>
<li class="show">Benzie, I. F., Strain, J. J., (1996). The ferric reducing ability of plasma (FRAP) as a measure of 'antioxidant power': the FRAP assay. Analytical biochemistry 239, 70–76. <a href="https://doi.org/10.1006/abio.1996.0292">https://doi.org/10.1006/abio.1996.0292</a></li>
<li class="show">Bozin, B., Mimica-Dukic, N.'Simin, N., Anackov, G., (2006). Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. Journal of agricultural and food chemistry 54, 1822–1828. <a href="https://doi.org/10.1021/jf051922u">https://doi.org/10.1021/jf051922u</a></li>
<li class="show">Chung, Y. C., Chang, C. T., Chao, W. W., Lin, C. F. Chou, S. T., (2002). Antioxidative activity and safety of the 50 ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. Journal of Agricultural and Food Chemistry 50, 2454–2458. <a href="https://doi.org/10.1021/jf011369q">https://doi.org/10.1021/jf011369q</a></li>
<li class="show">Deo, S. S., Inam, F.'Mahashabde, R. P., (2011). Antimicrobial activity and HPLC fingerprinting of crude ocimum extracts. Journal of Chemistry 8, 1430–1437.</li>
<li class="show">Devi, P. U., (2001). Radioprotective, anticarcinogenic and antioxidant properties of the Indian holy basil, Ocimum sanctum (Tulasi).</li>
<li class="show">Ito, N., Hirose, M., Fukushima, S., Tsuda, H., Shirai, T., Tatematsu, M., (1986). Studies on antioxidants: their carcinogenic and modifying effects on chemical carcinogenesis. Food and Chemical Toxicology 24, 1071–1082.<a href=" https://doi.org/10.1016/0278-6915(86)90291-7"> https://doi.org/10.1016/0278-6915(86)90291-7</a></li>
<li class="show">Katalinic, V., Milos, M., Kulisic, T., Jukic, M., (2006). Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food chemistry 94, 550–557. <a href="https://doi.org/10.1016/j.foodchem.2004.12.004">https://doi.org/10.1016/j.foodchem.2004.12.004</a></li>
<li class="show">Kumar, A., Agarwal, K., Maurya, A. K., Shanker, K., Bushra, U., Tandon, S., Bawankule, D. U., (2015). Pharmacological and phytochemical evaluation of Ocimum sanctum root extracts for its antiinflammatory, analgesic and antipyretic activities. Pharmacognosy magazine 11, S217. <a href="https://doi.org/10.4103/0973-1296.157743">https://doi.org/10.4103/0973-1296.157743</a></li>
<li class="show">Lagouri, V., Nisteropoulou, E., (2009). Antioxidant properties of O. onites, T. vulgaris and O. basilicum species grown in Greece and their total phenol and rosmarinic acid content. Journal of food Lipids 16, 484–498. <a href="https://doi.org/10.1111/j.1745-4522.2009.01161.x">https://doi.org/10.1111/j.1745-4522.2009.01161.x</a></li>
<li class="show">Luciana, M., Burgund, E. D., Berman, M., Hanson, K. L., (2001). Effects of tryptophan loading on verbal, spatial and affective working memory functions in healthy adults. Journal of Psychopharmacology 15, 219–230.<a href="https://doi.org/10.1177/026988110101500410">https://doi.org/10.1177/026988110101500410</a></li>
<li class="show">Luqman, S., Kumar, R., Kaushik, S., Srivastava, S., Darokar, M. P., Khanuja, S. P., (2009). Antioxidant potential of the root of Vetiveria zizanioides (L.) Nash. Indian J Biochem Biophys 46, 122–125.</li>
<li class="show">Luqman, S., Srivastava, S., Kumar, R., Maurya, A. K., Chanda, D., (2011). Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evidence-Based Complementary and Alternative Medicine Article ID 519084, 12 pages.</li>
<li class="show">Mabry, T. J., Markham, K. R.'Thomas, M. B., (1970). The ultraviolet spectra of flavones and flavonols. The systematic identification of flavonoids. Springer, pp. 41–164. <a href="https://doi.org/10.1007/978-3-642-88458-0_5">https://doi.org/10.1007/978-3-642-88458-0_5</a></li>
<li class="show">Maity, T. K., Mandal, S. C., Saha, B. P., Pal, M., (2000). Effect of Ocimum sanctum roots extract on swimming performance in mice. Phytotherapy Research 14, 120–121. <a href="https://doi.org/10.1002/(SICI)1099-1573(200003)14:2<120::AID-PTR557>3.3.CO;2-S">https://doi.org/10.1002/(SICI)1099-1573(200003)14:2<120::AID-PTR557>3.3.CO;2-S</a></li>
<li class="show">Markham, K. R., others, (1982). Techniques of flavonoid identification. Academic press London, Vol. 31.</li>
<li class="show">Meda, A., Lamien, C. E.'Romito, M., Millogo, J., Nacoulma, O. G., (2005). Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food chemistry 91, 571–577 <a href="https://doi.org/10.1016/j.foodchem.2004.10.006">https://doi.org/10.1016/j.foodchem.2004.10.006</a></li>
<li class="show">Negi, A. S., Luqman, S., Srivastava, S., Krishna, V., Gupta, N., Darokar, M. P., (2011). Antiproliferative and antioxidant activities of Juglans regia fruit extracts. Pharmaceutical biology 49, 669–673. <a href="https://doi.org/10.3109/13880209.2010.537666">https://doi.org/10.3109/13880209.2010.537666</a></li>
<li class="show">Pizzale, L., Bortolomeazzi, R., Vichi, S., Überegger, E., Conte, L. S., (2002). Antioxidant activity of sage (Salvia officinalis and S fruticosa) and oregano (Origanum onites and O indercedens) extracts related to their phenolic compound content. Journal of the Science of Food and Agriculture 82, 1645–1651. <a href="https://doi.org/10.1002/jsfa.1240">https://doi.org/10.1002/jsfa.1240</a></li>
<li class="show">Prieto, P., Pineda, M., Aguilar, M., (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical biochemistry 269, 337–341. <a href="https://doi.org/10.1006/abio.1999.4019">https://doi.org/10.1006/abio.1999.4019</a></li>
<li class="show">Singh, S. K., Anand, A. N., Verma, S. K., Siddiqui, M. A.'Mathur, A. B., Saklani, S. O., (2010). Analysis of phytochemical and antioxidant potential of Ocimum kilimandscharicum Linn. Int J Curr Pharm Res 3, 40–46.</li>
<li class="show">Singleton, V. L., Rossi, J. A., (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American journal of Enology and Viticulture 16, 144–158.</li>
<li class="show">Uyoh, E. A., Chukwurah, P. N.'David, I. A., Bassey, A. C., (2013). Evaluation of antioxidant capacity of two Ocimum species consumed locally as spices in Nigeria as a justification for increased domestication. American Journal of Plant Sciences 4, 222. <a href="https://doi.org/10.4236/ajps.2013.42029">https://doi.org/10.4236/ajps.2013.42029</a></li>
<li class="show">Vieira, P. R., de Morais, S. M.'Bezerra, F. H.'Ferreira, P. A. T.'Oliveira, Í. R.'Silva, M. G. V., (2014). Chemical composition and antifungal activity of essential oils from Ocimum species. Industrial Crops and Products 55, 267–271. <a href="https://doi.org/10.1016/j.indcrop.2014.02.032">https://doi.org/10.1016/j.indcrop.2014.02.032</a><br>Yen, G. C.Chen, H. Y., (1995). Antioxidant activity of various tea extracts in relation to their antimutagenicity. Journal of Agricultural and Food Chemistry 43, 27–32. https://doi.org/10.1021/jf00049a007</li>
</ol>
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
