Observation of TNF-α, IL-10 and HB-EGF gene expression by peripheral blood CD14+ mononuclear cells: a case of guttate psoriatic patient
DOI:
https://doi.org/10.15419/bmrat.v4i11.378Keywords:
CD14, guttate psoriasis, HB-EGF, IL-10, LPS, TNF-α, medicineAbstract
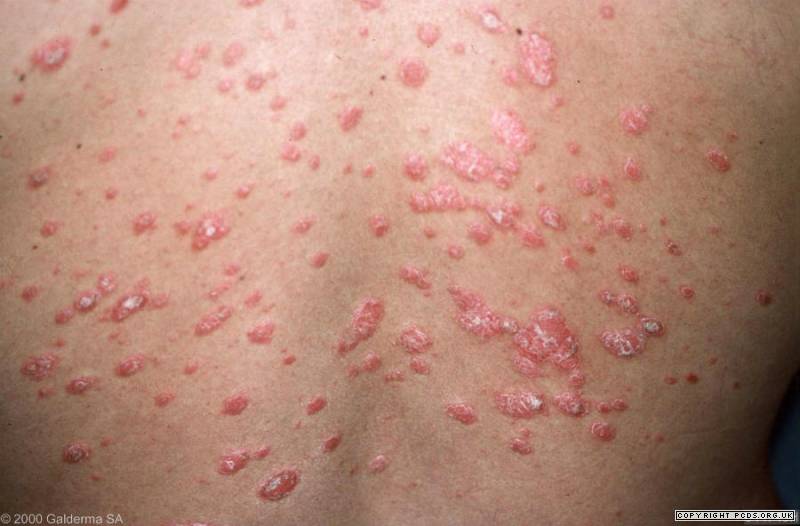
Introduction: Guttate is a type of psoriasis in which patients are sensitive to Streptococcus pneumoniae throughout innate immune responses. During the inflammation, tumour necrosis factor alpha (TNF-α), a well-known pro-inflammatory cytokine, is expressed; meanwhile interleukin 10 (IL-10) and heparin-binding EGF-like growth factor (HB-EGF), which are capable of inhibiting transcription of the TNF-α gene, are also prominent. Furthermore, HB-EGF only impacts fibroblasts and keratinocytes which promote psoriatic lesions. In this study, we looked for differences of TNF-α, IL-10 and HB-EGF expression between a psoriatic patient and a non-psoriatic relative.
Methods: To achieve our target, peripheral blood mononuclear cells (PBMCs) expressing LPS receptors or CD14 (CD14+ cells) derived from a guttate patient, and the donor’s father (without psoriatic symptoms), were activated for 7 days by a lysate of Streptococcus pneumoniae for 24 hours before being harvested.
Results: Results showed detectable mRNAs of TNF-α, IL-10 and HB-EGF from isolated CD14+ cells of guttate patient were more intensive expression than the non-psoriatic one at 24 hours after engaging the bacterial components. In addition, transcription of HB-EGF gene from the guttate patient was maintained over 168 hours, while its mRNA level from the non-psoriatic volunteer was only expressed within 24 hours.
Conclusion: Finally, in initial results of inflammatory effects between strains, the Streptococcal lysate was seen to have stronger immune responses than the Staphylococcal lysate on the immune cells of the guttate psoriasis.
References
<li class="show">Anderson, K., Petersson, S., Wong, J., Shubbar, E., Lokko, N., Carlström, M., & Enerbäck, C. (2010). Elevation of serum epidermal growth factor and interleukin 1 receptor antagonist in active psoriasis vulgaris. <em>British Journal of Dermatology</em>, 163(5), 1085–1089. <a href="https://doi.org/10.1111/j.1365-2133.2010.09990.x">https://doi.org/10.1111/j.1365-2133.2010.09990.x</a></li>
<li class="show">Arias-Santiago, S., Espiñeira-Carmona, M. J., & Aneiros-Fernández, J. (2013). The Koebner phenomenon: Psoriasis in tattoos. <em>Canadian Medical Association Journal</em>, 185(7), 585–585. <a href="https://doi.org/10.1503/cmaj.111299">https://doi.org/10.1503/cmaj.111299</a></li>
<li class="show">Baek, M. K., Kim, M. H., Jang, H. J., Park, J. S., Chung, I. J., Shin, B. A., . . . Jung, Y. D. (2008). EGF stimulates uPAR expression and cell invasiveness through ERK, AP-1, and NF-κB signaling in human gastric carcinoma cells. <em>Oncology Reports</em>, 20, 1569–1575.</li>
<li class="show">Balato, A., Lembo, S., Mattii, M., Schiattarella, M., Marino, R., Paulis, A., . . . Ayala, F. (2012). IL‐33 is secreted by psoriatic keratinocytes and induces pro‐inflammatory cytokines via keratinocyte and mast cell activation. <em>Experimental Dermatology</em>, 21(11), 892–894. <a href="https://doi.org/10.1111/exd.12027">https://doi.org/10.1111/exd.12027</a></li>
<li class="show">Baurecht, H., Hotze, M., Brand, S., Büning, C., Cormican, P., Corvin, A., . . . Fölster-Holst, R. (2015). Genome-wide Comparative Analysis of Atopic Dermatitis and Psoriasis Gives Insight into Opposing Genetic Mechanisms. <em>American Journal of Human Genetics</em>, 96(1), 104–120. <a href="https://doi.org/10.1016/j.ajhg.2014.12.004">https://doi.org/10.1016/j.ajhg.2014.12.004</a></li>
<li class="show">Bhardwaj, R. S., Schwarz, A., Becher, E., Mahnke, K., Aragane, Y., Schwarz, T., & Luger, T. A. (1996). Pro-opiomelanocortin-derived peptides induce IL-10 production in human monocytes. <em>Journal of Immunology (Baltimore, Md.: 1950)</em>, 156, 2517–2521.</li>
<li class="show">Byrne, A., & Reen, D. J. (2002). Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils.<em> Journal of Immunology (Baltimore, Md.: 1950)</em>, 168(4), 1968–1977. <a href="https://doi.org/10.4049/jimmunol.168.4.1968">https://doi.org/10.4049/jimmunol.168.4.1968</a></li>
<li class="show">Chiricozzi, A., Guttman-Yassky, E., Suárez-Farinas, M., Nograles, K. E., Tian, S., Cardinale, I., . . . Krueger, J. G. (2011). Integrative responses to IL-17 and TNF-α in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. <em>The Journal of Investigative Dermatology</em>, 131(3), 677–687. <a href="https://doi.org/10.1038/jid.2010.340">https://doi.org/10.1038/jid.2010.340</a></li>
<li class="show">Dugas, N., Vouldoukis, I., Bécherel, P., Arock, M., Debré, P., Tardieu, M., . . . Dugas, B. (1996). Triggering of CD23b antigen by anti‐CD23 monoclonal antibodies induces interleukin‐10 production by human macrophages. <em>European Journal of Immunology</em>, 26(6), 1394–1398. <a href="https://doi.org/10.1002/eji.1830260632 ">https://doi.org/10.1002/eji.1830260632 </a></li>
<li class="show">Evans, H. G., Gullick, N. J., Kelly, S., Pitzalis, C., Lord, G. M., Kirkham, B. W., & Taams, L. S. (2009). In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. <em>Proceedings of the National Academy of Sciences of the United States of America</em>, 106(15), 6232–6237. <a href="https://doi.org/10.1073/pnas.0808144106">https://doi.org/10.1073/pnas.0808144106</a></li>
<li class="show">Evans, P., Ovaa, H., Hamon, M., Kilshaw, P., Hamm, S., Bauer, S., . . . Smith, T. (2004). Zinc-finger protein A20, a regulator of inflammation and cell survival, has de-ubiquitinating activity. <em>The Biochemical Journal</em>, 378(3), 727–734. <a href="https://doi.org/10.1042/bj20031377">https://doi.org/10.1042/bj20031377</a></li>
<li class="show">Gervin, K., Vigeland, M. D., Mattingsdal, M., Hammero, M., Nygard, H., Olsen, A. O., . . . Lyle, R. (2012). DNA methylation and gene expression changes in monozygotic twins discordant for psoriasis: Identification of epigenetically dysregulated genes. <em>PLOS Genetics</em>, 8(1), e1002454. <a href="https://doi.org/10.1371/journal.pgen.1002454">https://doi.org/10.1371/journal.pgen.1002454</a></li>
<li class="show">Gratchev, A., Kzhyshkowska, J., Kannookadan, S., Ochsenreiter, M., Popova, A., Yu, X., . . . Gooi, L. (2008). Activation of a TGF-β-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-β receptor II. <em>Journal of Immunology</em> (Baltimore, Md.: 1950), 180(10), 6553–6565. <a href="https://doi.org/10.4049/jimmunol.180.10.6553">https://doi.org/10.4049/jimmunol.180.10.6553</a></li>
<li class="show">Gudjonsson, J., Thorarinsson, A., Sigurgeirsson, B., Kristinsson, K., & Valdimarsson, H. (2003). Streptococcal throat infections and exacerbation of chronic plaque psoriasis: A prospective study. <em>British Journal of Dermatology</em>, 149(3), 530–534. <a href="https://doi.org/10.1046/j.1365-2133.2003.05552.x">https://doi.org/10.1046/j.1365-2133.2003.05552.x</a></li>
<li class="show">Haider, A. S., Lowes, M. A., Suárez-Fariñas, M., Zaba, L. C., Cardinale, I., Khatcherian, A., . . . Krueger, J. G. (2008). Identification of cellular pathways of “type 1,” Th17 T cells, and TNF-and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. <em>Journal of Immunology</em> (Baltimore, Md.: 1950), 180(3), 1913–1920. <a href="https://doi.org/10.4049/jimmunol.180.3.1913">https://doi.org/10.4049/jimmunol.180.3.1913</a></li>
<li class="show">Inoue, M., Arikawa, T., Chen, Y.-H., Moriwaki, Y., Price, M., Brown, M., . . . Shinohara, M. L. (2014). T cells down-regulate macrophage TNF production by IRAK1-mediated IL-10 expression and control innate hyperinflammation. <em>Proceedings of the National Academy of Sciences of the United States of America</em>, 111(14), 5295–5300. <a href="https://doi.org/10.1073/pnas.1321427111 ">https://doi.org/10.1073/pnas.1321427111 </a></li>
<li class="show">Johnson, G. R., Kannan, B., Shoyab, M., & Stromberg, K. (1993). Amphiregulin induces tyrosine phosphorylation of the epidermal growth factor receptor and p185erbB2. Evidence that amphiregulin acts exclusively through the epidermal growth factor receptor at the surface of human epithelial cells. <em>The Journal of Biological Chemistry</em>, 268, 2924–2931.</li>
<li class="show">Johnston, A., Gudjonsson, J., Sigmundsdottir, H., Love, T., & Valdimarsson, H. (2004). Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin‐homing CD8+ T cells. <em>Clinical and Experimental Immunology</em>, 138(1), 83–93. <a href="https://doi.org/10.1111/j.1365-2249.2004.00600.x ">https://doi.org/10.1111/j.1365-2249.2004.00600.x </a></li>
<li class="show">Jordan, C. T., Cao, L., Roberson, E. D., Duan, S., Helms, C. A., Nair, R. P., . . . Hayashi, G. (2012). Rare and common variants in CARD14, encoding an epidermal regulator of NF-kappaB, in psoriasis. <em>American Journal of Human Genetics</em>, 90(5), 796–808. <a href="https://doi.org/10.1016/j.ajhg.2012.03.013 ">https://doi.org/10.1016/j.ajhg.2012.03.013 </a></li>
<li class="show">Kumari, S., Bonnet, M. C., Ulvmar, M. H., Wolk, K., Karagianni, N., Witte, E., . . . Toftgard, R. (2013). Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. <em>Immunity</em>, 39(5), 899–911. <a href="https://doi.org/10.1016/j.immuni.2013.10.009">https://doi.org/10.1016/j.immuni.2013.10.009</a></li>
<li class="show">Maheshwari, A., Kelly, D. R., Nicola, T., Ambalavanan, N., Jain, S. K., Murphy–Ullrich, J., . . . Aprahamian, C. (2011). TGF-β 2 Suppresses Macrophage Cytokine Production and Mucosal Inflammatory Responses in the Developing Intestine. <em>Gastroenterology</em>, 140(1), 242–253. <a href="https://doi.org/10.1053/j.gastro.2010.09.043">https://doi.org/10.1053/j.gastro.2010.09.043</a></li>
<li class="show">Nair, R. P., Stuart, P. E., Nistor, I., Hiremagalore, R., Chia, N. V., Jenisch, S., . . . Christophers, E. (2006). Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. <em>American Journal of Human Genetics</em>, 78(5), 827–851. <a href="https://doi.org/10.1086/503821">https://doi.org/10.1086/503821</a></li>
<li class="show">Poumay, Y., & De Rouvroit, C. L. (2012). HB-EGF, the growth factor that accelerates keratinocyte migration, but slows proliferation. <em>The Journal of Investigative Dermatology</em>, 132(9), 2129–2130. <a href="https://doi.org/10.1038/jid.2012.225">https://doi.org/10.1038/jid.2012.225</a></li>
<li class="show">Rocourt, D. V., Mehta, V. B., & Besner, G. E. (2007). Heparin-binding EGF-like growth factor decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury. <em>The Journal of Surgical Research</em>, 139(2), 269–273. <a href="https://doi.org/10.1016/j.jss.2006.10.047">https://doi.org/10.1016/j.jss.2006.10.047</a></li>
<li class="show">Saraiva, M., Christensen, J. R., Tsytsykova, A. V., Goldfeld, A. E., Ley, S. C., Kioussis, D., & Anne, O. (2005). Identification of a macrophage-specific chromatin signature in the IL-10 locus. <em>Journal of Immunology</em> (Baltimore, Md.: 1950), 175(2), 1041–1046. <a href="https://doi.org/10.4049/jimmunol.175.2.1041">https://doi.org/10.4049/jimmunol.175.2.1041</a></li>
<li class="show">Sato, K., Takaishi, M., Tokuoka, S., & Sano, S. (2015). Correction: Involvement of TNF-α Converting Enzyme in the Development of Psoriasis-Like Lesions in a Mouse Model. <em>PLoS One</em>, 10(4), e0124989. <a href="https://doi.org/10.1371/journal.pone.0124989">https://doi.org/10.1371/journal.pone.0124989</a></li>
<li class="show">Shembade, N., Ma, A., & Harhaj, E. W. (2010). Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. <em>Science</em>, 327(5969), 1135–1139. <a href="https://doi.org/10.1126/science.1182364">https://doi.org/10.1126/science.1182364</a></li>
<li class="show">Shirakata, Y., Kimura, R., Nanba, D., Iwamoto, R., Tokumaru, S., Morimoto, C., . . . Mekada, E. (2005). Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. <em>Journal of Cell Science</em>, 118(11), 2363–2370. <a href="https://doi.org/10.1242/jcs.02346">https://doi.org/10.1242/jcs.02346</a></li>
<li class="show">Thorarensen, S., Lu, N., Ogdie, A., Gelfand, J., Choi, H., & Love, T. (2015). OP0311 Physical Trauma is Associated with the Onset of Psoriatic Arthritis Among Psoriasis Patients. <em>Annals of the Rheumatic Diseases</em>, 74(Suppl 2), 190–191.<a href=" https://doi.org/10.1136/annrheumdis-2015-eular.3297"> https://doi.org/10.1136/annrheumdis-2015-eular.3297</a></li>
<li class="show">Tsoi, L. C., Spain, S. L., Knight, J., Ellinghaus, E., Stuart, P. E., Capon, F., . . . Gudjonsson, J. E. (2012). Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. <em>Nature Genetics</em>, 44(12), 1341–1348. <a href="https://doi.org/10.1038/ng.2467">https://doi.org/10.1038/ng.2467</a></li>
<li class="show">Volpe, E., Pattarini, L., Martinez-Cingolani, C., Meller, S., Donnadieu, M.-H., Bogiatzi, S.I., Fernandez, M.I., Touzot, M., Bichet, J.-C., and Reyal, F. (2014). Thymic stromal lymphopoietin links keratinocytes and dendritic cell–derived IL-23 in patients with psoriasis. <em>Journal of Allergy and Clinical Immunology</em> 134, 373-381. e374.</li>
<li class="show">Wang, B., Rao, Y.-H., Inoue, M., Hao, R., Lai, C.-H., Chen, D., . . . Shinohara, M. L. (2014). Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. <em>Nature Communications</em>, 5, 3479–3479.</li>
<li class="show">Wang, H., Peters, T., Kess, D., Sindrilaru, A., Oreshkova, T., Van Rooijen, N., . . . Wlaschek, M. (2006). Activated macrophages are essential in a murine model for T cell–mediated chronic psoriasiform skin inflammation. <em>The Journal of Clinical Investigation</em>, 116(8), 2094–2105. <a href="https://doi.org/10.1172/JCI27180">https://doi.org/10.1172/JCI27180</a></li>
<li class="show">Yoshizumi, M., Kourembanas, S., Temizer, D., Cambria, R., Quertermous, T., & Lee, M.-E. (1992). Tumor necrosis factor increases transcription of the heparin-binding epidermal growth factor-like growth factor gene in vascular endothelial cells. <em>The Journal of Biological Chemistry</em>, 267, 9467–9469.</li>
<li class="show">Yotsumoto, F., Oki, E., Tokunaga, E., Maehara, Y., Kuroki, M., & Miyamoto, S. (2010). HB‐EGF orchestrates the complex signals involved in triple‐negative and trastuzumab‐resistant breast cancer. <em>International Journal of Cancer</em>, 127(11), 2707–2717. <a href="https://doi.org/10.1002/ijc.25472">https://doi.org/10.1002/ijc.25472</a></li>
</ol>
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
