Engineered cartilage tissue from biodegradable Poly(ε-caprolactone) scaffold and human umbilical cord derived mesenchymal stem cells
DOI:
https://doi.org/10.15419/bmrat.v5i02.414Keywords:
3D scaffold, Chondrocytes, Human umbilical cord, Mesenchymal stem cells, Poly(ε-caprolactone) (PCL)Abstract
Introduction: Cartilage injury is the most common injury among orthopedic diseases. The predominant treatment for this condition is cartilage transplantation. Therefore, production of cartilage for treatment is an important strategy in regenerative medicine of cartilage to provide surgeons with an additional option for treatment of cartilage defects. This study aimed to produce in vitro engineered cartilage tissue by culturing and differentiating umbilical cord derived mesenchymal stem cells on biodegradable Poly(ε-caprolactone) (PCL) scaffold.
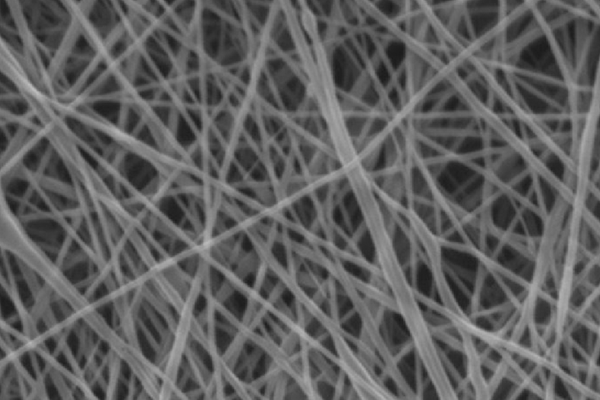
Methods: Human umbilical cord derived mesenchymal stem cells (UCMSCs) were isolated and expanded according to previous published protocols. UCMSCs were labeled with CD90 APC‑conjugated monoclonal antibody (CD90-APC) and then seeded onto porous PCL scaffolds. Cell adhesion and proliferation on PCL scaffolds were evaluated based on the strength/signal of APC, MTT assays, and scanning electron microscopy (SEM). The chondrogenic differentiation of UCMSCs on scaffolds was detected by Alcian Blue and Safranin O staining.
Results: The results showed that UCMSCs successfully adhered, proliferated and differentiated into chondroblasts and chondrocytes on PCL scaffolds. The chondrocyte scaffolds were positive for some markers of cartilage, as indicated by Alcian Blue and Safranin O staining.
Conclusion: In conclusion, this study showed successful production of cartilage tissues from UCMSCs on PCL scaffolds.
References
Bui, K.H.-T., Duong, T.D., Nguyen, N.T., Nguyen, T.D., Le, V.T., Mai, V.T., Phan, N.L.-C., Le, D.M., Phan, N.K., and Van Pham, P. (2014). Symptomatic knee osteoarthritis treatment using autologous adipose derived stem cells and platelet-rich plasma: a clinical study. Biomedical Research and Therapy 1, 02-08. https://doi.org/10.15419/bmrat.v1i01.11
Demoor, M., Ollitrault, D., Gomez-Leduc, T., Bouyoucef, M., Hervieu, M., Fabre, H., . . . Galera, P. (2014). Cartilage tissue engineering: Molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochimica et Biophysica Acta, 1840(8), 2414–2440. https://doi.org/10.1016/j.bbagen.2014.02.030 PMID:24608030
Ding, D.-C., Chang, Y.-H., Shyu, W.-C., & Lin, S.-Z. (2015). Human umbilical cord mesenchymal stem cells: A new era for stem cell therapy. Cell Transplantation, 24(3), 339–347. https://doi.org/10.3727/096368915X686841 PMID:25622293
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., . . . Horwitz, E. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. https://doi.org/10.1080/14653240600855905 PMID:16923606
Gauthaman, K., Venugopal, J. R., Yee, F. C., Biswas, A., Ramakrishna, S., & Bongso, A. (2011). Osteogenic differentiation of human Wharton’s jelly stem cells on nanofibrous substrates in vitro. Tissue Engineering. Part A, 17(1-2), 71–81. https://doi.org/10.1089/ten.tea.2010.0224 PMID:20673136
Guarino, V., Alvarez-Perez, M., Cirillo, V., & Ambrosio, L. (2011). hMSC interaction with PCL and PCL/gelatin platforms: A comparative study on films and electrospun membranes. Journal of Bioactive and Compatible Polymers, 26(2), 144–160. https://doi.org/10.1177/0883911511399410
Hangody, L., Kish, G., Karpati, Z., Szerb, I., and Udvarhelyi, I. (1997). Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA 5, 262-267.
Labet, M., & Thielemans, W. (2009). Synthesis of polycaprolactone: A review. Chemical Society Reviews, 38(12), 3484–3504. https://doi.org/10.1039/b820162p PMID:20449064
Marmotti, A., Mattia, S., Castoldi, F., Barbero, A., Mangiavini, L., Bonasia, D. E., . . . Peretti, G. M. (2017). Allogeneic Umbilical Cord-Derived Mesenchymal Stem Cells as a Potential Source for Cartilage and Bone Regeneration: An In Vitro Study. Stem Cells International, 2017, 1732094. https://doi.org/10.1155/2017/1732094 PMID:29358953
Nguyen, P. D., Tran, T. D., Nguyen, H. T., Vu, H. T., Le, P. T., Phan, N. L., . . . Van Pham, P. (2017). Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Translational Medicine, 6(1), 187–195. https://doi.org/10.5966/sctm.2016-0023 PMID:28170179
Nirmal, R. S., & Nair, P. D. (2013). Significance of soluble growth factors in the chondrogenic response of human umbilical cord matrix stem cells in a porous three dimensional scaffold. European Cells & Materials, 26, 234–251. https://doi.org/10.22203/eCM.v026a17 PMID:24213879
Okamoto, Y., Nakagawa, Y., Maekawa, M., Kobayashi, M., and Nakamura, T. (2007). Osteochondral grafting for treatment of a massive chondral defect in the knee of a young adult with anterior cruciate ligament deficit. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association 23, 1024.e1021-1024.
Podskubka, A., Povýsil, C., Kubes, R., Sprindrich, J., & Sedlácek, R. (2006). [Treatment of deep cartilage defects of the knee with autologous chondrocyte transplantation on a hyaluronic Acid ester scaffolds (Hyalograft C)]. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca, 73(4), 251–263. PMID:17026884
Sledge, S. L. (2001). Microfracture techniques in the treatment of osteochondral injuries. Clinics in Sports Medicine, 20(2), 365–377. https://doi.org/10.1016/S0278-5919(05)70311-2 PMID:11398363
Steadman, J. R., Rodkey, W. G., Briggs, K. K., & Rodrigo, J. J. (1999). [The microfracture technic in the management of complete cartilage defects in the knee joint]. Der Orthopade, 28(1), 26–32. PMID:10081041
Van Pham, P., Truong, N. C., Le, P. T.-B., Tran, T. D.-X., Vu, N. B., Bui, K. H.-T., & Phan, N. K. (2016a). Isolation and proliferation of umbilical cord tissue derived mesenchymal stem cells for clinical applications. Cell and Tissue Banking, 17(2), 289–302. https://doi.org/10.1007/s10561-015-9541-6 PMID:26679929
Van Pham, P., Vu, N. B., & Phan, N. K. (2016b). Umbilical cord-derived stem cells (MODULATISTTM) show strong immunomodulation capacity compared to adipose tissue-derived or bone marrow-derived mesenchymal stem cells. Biomedical Research and Therapy, 3(6), 687–696. https://doi.org/10.7603/s40730-016-0029-1
Woodruff, M. A., & Hutmacher, D. W. (2010). The return of a forgotten polymer—Polycaprolactone in the 21st century. Progress in Polymer Science, 35(10), 1217–1256. https://doi.org/10.1016/j.progpolymsci.2010.04.002
Xu, J., Wang, W., Ludeman, M., Cheng, K., Hayami, T., Lotz, J. C., & Kapila, S. (2008). Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Engineering. Part A, 14(5), 667–680. https://doi.org/10.1089/tea.2007.0272 PMID:18377198
Xue, R., Qian, Y., Li, L., Yao, G., Yang, L., & Sun, Y. (2017). Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells. Stem Cell Research & Therapy, 8(1), 148. https://doi.org/10.1186/s13287-017-0588-0 PMID:28646917
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
