Complete response of Palbociclib in metastatic breast cancer patient: A case report
DOI:
https://doi.org/10.15419/bmrat.v5i6.448Abstract
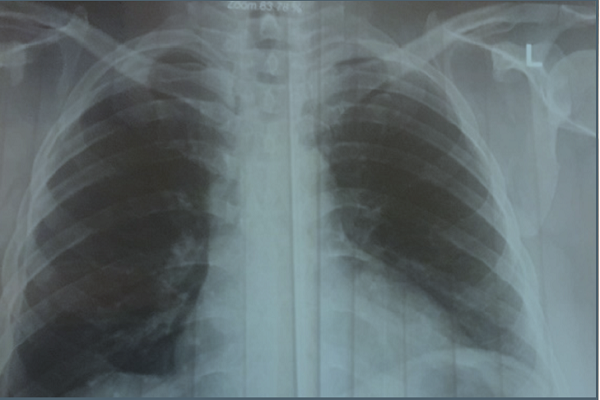
Palbociclib, an oral inhibitor of cyclin-dependent kinases 4 and 6 (CDK 4/6), has been approved for metastatic breast cancer (mBC) treatment of hormone receptor (HR)-positive/ human epidermal growth factor receptor 2 (HER2)-negative. The study reported the efficacy of Palbociclib as a new oral drug in a patient with mBC. A 40-year-old female with stage 2 right BC change to stage 4 after about two years later referred to oncology clinic. Due to HR-positivity/HER2-negative, she has treated with Palbociclib 125 mg (per one day for twoweek and one-week intervals) with Letrozole. In new assessment and after 8 months of this oral combination therapy, the chest x-ray of lung showed the complete response. Treatment with Palbociclib plus Letrozole had a complete response in the mBC patient after the common chemotherapies and hormone monotherapy.
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
