Evaluation of the efficacy of recombinant tissue plasminogen activators in improving clinical status of acute ischemic stroke: A randomized clinical trial in Iran
DOI:
https://doi.org/10.15419/bmrat.v5i9.481Keywords:
Acute ischemic stroke, Iran, Neurologic disability, Randomized clinical trial, Recombinant tissue plasminogen activatorAbstract
Background: Acute ischemic stroke is caused by blockage of a cerebral artery and is also known as cerebrovascular accident (CVA). Recombinant tissue plasminogen activator (rt-PA) therapy is effective in reducing early and long-term neurologic disabilities if it is started quickly. Therefore, the aim of this study was to determine the efficacy of treatment with recombinant tissue plasminogen activators in improving the clinical status of acute ischemic stroke.
Methods: The current study was performed as a clinical trial of two groups- treatment and control (n=20 per group). The treatment group consisted of patients who received rt-PA, while the control group consisted of patients who did not receive rt-PA. For each group, evaluation of neurological disabilities following ischemic stroke was based off the National Institutes of Health Stroke Scale (NIHSS), for early assessment of disability on the third day of treatment), and off the modified Rankin Scale (MRS) at 90 days after stroke. The drug effect criterion was used to reduce the neurological disability or the difference in NIHSS on day 3 after treatment. Also, the duration of onset of symptoms until the arrival of the patients to the emergency room (ER), as well as the risk factors, complications and number of deaths in both groups, were recorded. Data obtained were analyzed by SPSS software.
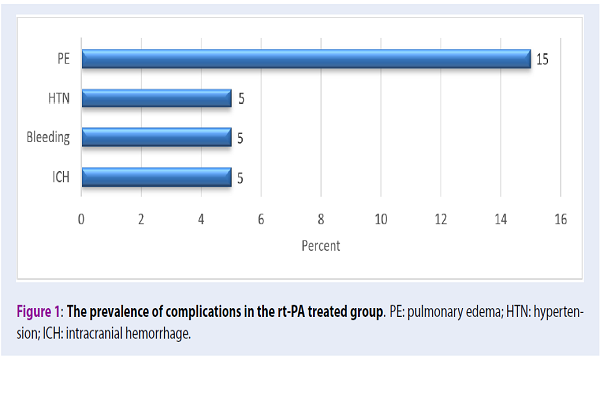
Results: The results of the study showed that the mean of ER arrival time, NIHSS before treatment, and NIHSS on day 3 of treatment in the control group was higher than that of the treatment group; the difference was statistically significant (P<0.05). The results also indicated that the probability of improvement of neurological disabilities in the experimental group was greater than that of the control group (relative risk (RR) =1.25). Additionally, the odds ratio (OR) for receiving rt-PA in the NIHSS positive treatment group compared to the control group was equal to 1/5 (OR=1.5). The results showed that 30% of the patients in the treatment group were treated with a complication. The mean of MRS was higher in the control group at 90 days after the stroke, compared with the treatment group.
Conclusion: Treatment with rt-PA reduces the neurological disability in patients with ischemic stroke, since the mean of MRS is lower in the treatment group, compared with the control group, after 90 days of treatment.
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
