Efficacy of adding Luvos® Healing Earth supplementation to mebeverine in improving symptoms and quality of life of patients with diarrhea-predominant irritable bowel syndrome: A randomized clinical trial
DOI:
https://doi.org/10.15419/bmrat.v5i10.493Keywords:
Diarrhea, Irritable bowel syndrome, Mebeverine, Quality of life, Supplementation, TreatmentAbstract
Introduction: Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder. Treatment can improve symptoms and social functioning in the patients. This study was designed to assess the effect of adding Luvos supplementation to mebeverine on improving symptoms and quality of life (QOL) in patients with diarrhea-predominant irritable bowel syndrome.
Methods: Eighty patients with diarrhea-predominant IB, ages 18-65, were diagnosed by the Rome IV criteria and randomly assigned to the study. Forty patients (group A) received mebeverine (135 mg) twice a day (bid) plus Luvos®Healing Earth (1 sachet, bid). The other 41 patients (group B) received mebeverine (135 mg) bid for 4 weeks. Basic demographic data, Bristol score, symptom severity score, and QOL questionnaire were recorded at the start and completion of treatment. The data were analyzed by SPSS version 22.
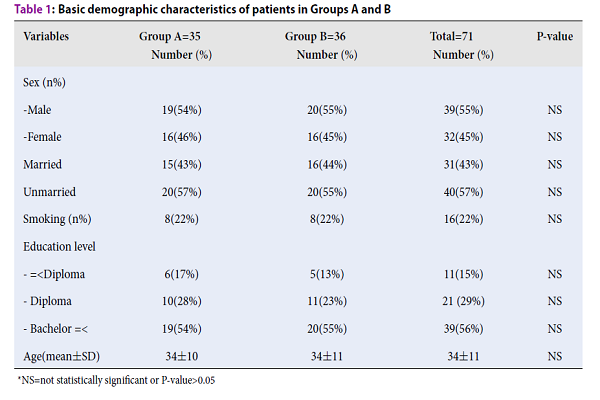
Results: Seventy one of the patients (35 and 36 patients in groups A and B, respectively) completed the study. The majority of the patients were young males, unmarried and highly educated. Diarrhea and QOL were both significantly improved in group A when compared to group B (P=0.036 and P=0.028, respectively). We did not find a significant difference (improvement) in abdominal pain or overall symptom score between group A (mebeverine + Luvos) compared to group B (mebeverine alone) (P=0.096 and P=0.071, respectively). Mild and tolerable adverse effects were observed in 2.8% (2/71) of the patients.
Conclusion: According to our results, Luvos supplementation is safe, effective and well-tolerated in diarrhea-predominant irritable bowel syndrome patients. Further study with a larger sample size is recommended to evaluate the efficacy of this natural clay-like medicine.
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
