A randomized, double-blinded, controlled trial of herbal medicine combination for leg symptoms due to chronic venous disease
DOI:
https://doi.org/10.15419/bmrat.v6i2.523Keywords:
Acemannan, Acetylsalicylic acid, Asiaticoside, Chronic venous disease, Double blinded Randomized Control Trial, Herbal medicine combination, Leg symptomsAbstract
Background: The herbal medicine combination (HMC) containing 2% Asiaticoside and 1% Acemannan, in a Beeswax encapsulation of 2% Acetylsalicylic acid base, was developed as a topical treatment option for mild-to-moderate chronic venous disease (CVD). For control (C), Beeswax encapsulation of 2% Acetylsalicylic acid base was used.
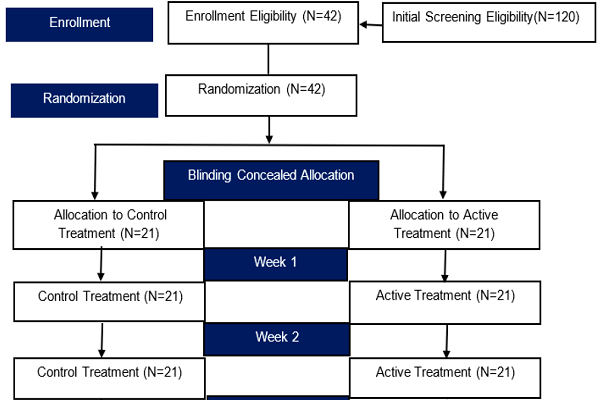
Methods: A double-blinded, randomized controlled trial comparing HMC with C was investigated among 42 CVD patients; 21 patients was allocated to receive either HMC or C, for a continuous 12-week treatment. To assess the efficacy, the following were employed: (i) Venous Clinical Severity Score (VCSS), (ii) Patients Self-Rating Symptoms Score (PSSS), and (iii) Short-form health-related quality of life score based on a Medical Outcomes Study 14-item Chronic Venous Disease (MOS CIVIQ14). The short-term safety was assessed by the patients' self-reported adverse events. The primary endpoint was a responder rate defined by a 50% reduction of the VCSS from baseline after 4 weeks of treatment. The secondary endpoints were assessed by improvement of overall disability after employing VCSS, PSSS, and MOS CIVIQ14, accordingly.
Results: CVD Thai patients (N=42), regardless of clinical severity of CEAP class (CEAP1/CEAP2)or co-morbidity, responded 57.14% to HMC (N=21) as compared with 9.52% to C (N=21) the responder rate was significantly dependent on intervention (p=0.003 and p=0.003, respectively). HMC reduced VCSS markedly better than C, over the 4 weeks: 11.9048 (6.4908) vs. 22.4702 (7.9438), p<0.001. HMC reduced VCSS and PSSS scores significantly better than C did: from baseline to 2 weeks (p=0.001 vs. p=0.017, respectively), from 2 to 4 weeks (p=0.001 vs. p=0.004, respectively), and from 8 to12 weeks (p=0.001 vs. p=0.003, respectively). After 12 weeks from baseline, from both the global and subscores for MOS CIVIQ14, treatment with HMC and C significantly improved scores (p<0.001 and p<0.001, respectively). For the adverse drug events reported, there were no statistically significantly differences between HMC and C from baseline to the end of the 12-week follow-up (p=0.756).
Conclusion: The herbal medicine combination (HMC), consisting of 2% Asiaticoside and 1% Acemannan in Beeswax encapsulation of 2% Acetylsalicylic acid, as a topical administration in optional medical management for Thai patients with leg symptoms due to chronic venous disease, provided a clinical responder rate that was better than that of control. In terms of short-term safety assessments, no significant statistical differences for adverse events were reported with regards to incidence for both HMC and C treatments. Further studies are warranted to explore and develop this herbal medicine base combination.
Trial registration no.: ISRCTN54360155
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
