Preclinical toxicological evaluation of measles virus vaccine strain in non-human primates: A two-month intravenous study
DOI:
https://doi.org/10.15419/bmrat.v8i6.676Keywords:
concentrated measles vaccine, Macaca mulata monkey;, preclinical toxicologyAbstract
Introduction: Based on its ability to kill tumor cells, the vaccine strain of the measles virus is used for oncolytic virotherapy. However, the dose required for cancer therapy is much higher than that used for vaccination. Therefore, this study was conducted to evaluate the preclinical toxicology of the vaccine strain of measles virus in monkeys.
Methods: 16 healthy Macaca mulata monkeys were randomly divided into four groups, of which one was a control. A preclinical safety evaluation of the vaccine strain of the measles virus was performed, and the three experimental groups were intravenously injected with the strain at doses of 105 TCID50, 106 TCID50 and 107 TCID50 respectively.
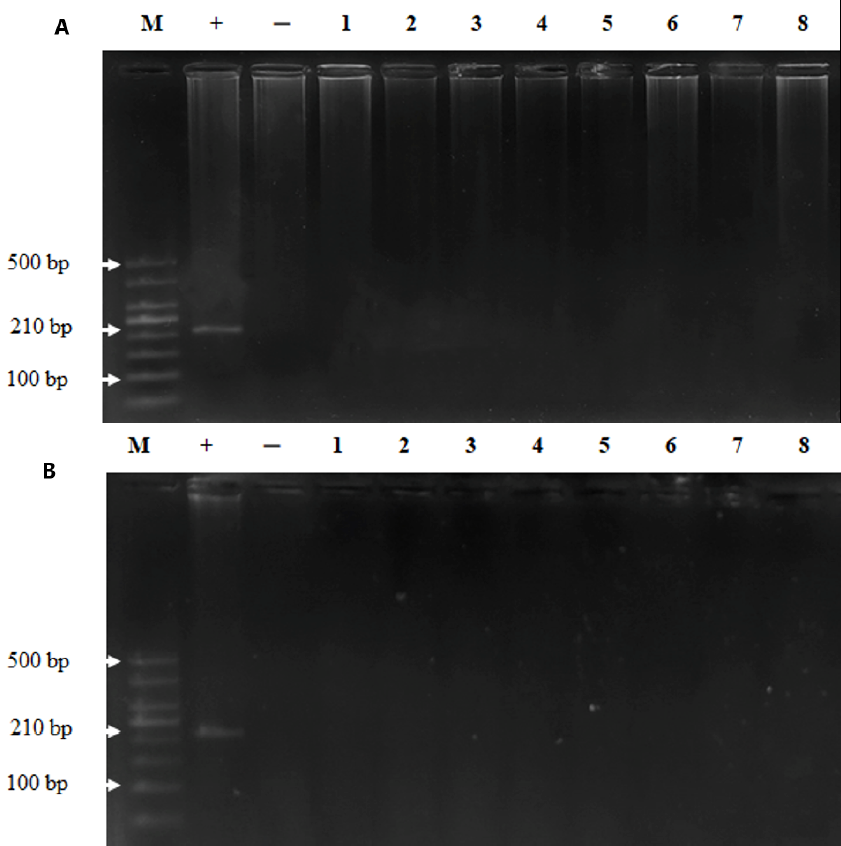
Results: There were no significant abnormalities in the physical, clinical, haematological, and biochemical parameters following the intravenous injection with measles vaccine at doses of 105 TCID50, 106 TCID50 and 107 TCID50. The vaccine strain of measles virus remained in the plasma until the 30th day and disappeared on the 60th, and it did not persist in the tissues on days 30 and 60 post injection. Measles IgG antibody was negative on days 0, 1, 3, and 8 and was positive on days 15, 30, and 60 post administration of the measles virus. The histopathology of target organs was not affected in all groups on days 30 and 60 post injection.
Conclusions: The systematic preclinical safety data of the present study confirms the safety of two months of concentrated measles vaccine administration in the Macaca mulata monkey for clinical trials.
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
