Umbilical Cord Tissue-derived Mesenchymal Stem Cells Should be Considered as Adjuvant Therapy for COVID-19 Treatment: An Opinion from Pooled Clinical Evidence
DOI:
https://doi.org/10.15419/bmrat.v8i9.694Keywords:
Covid-19, Mesenchymal stem cell, Stem cells, Umbilical cord derived mesenchymal stem cellAbstract
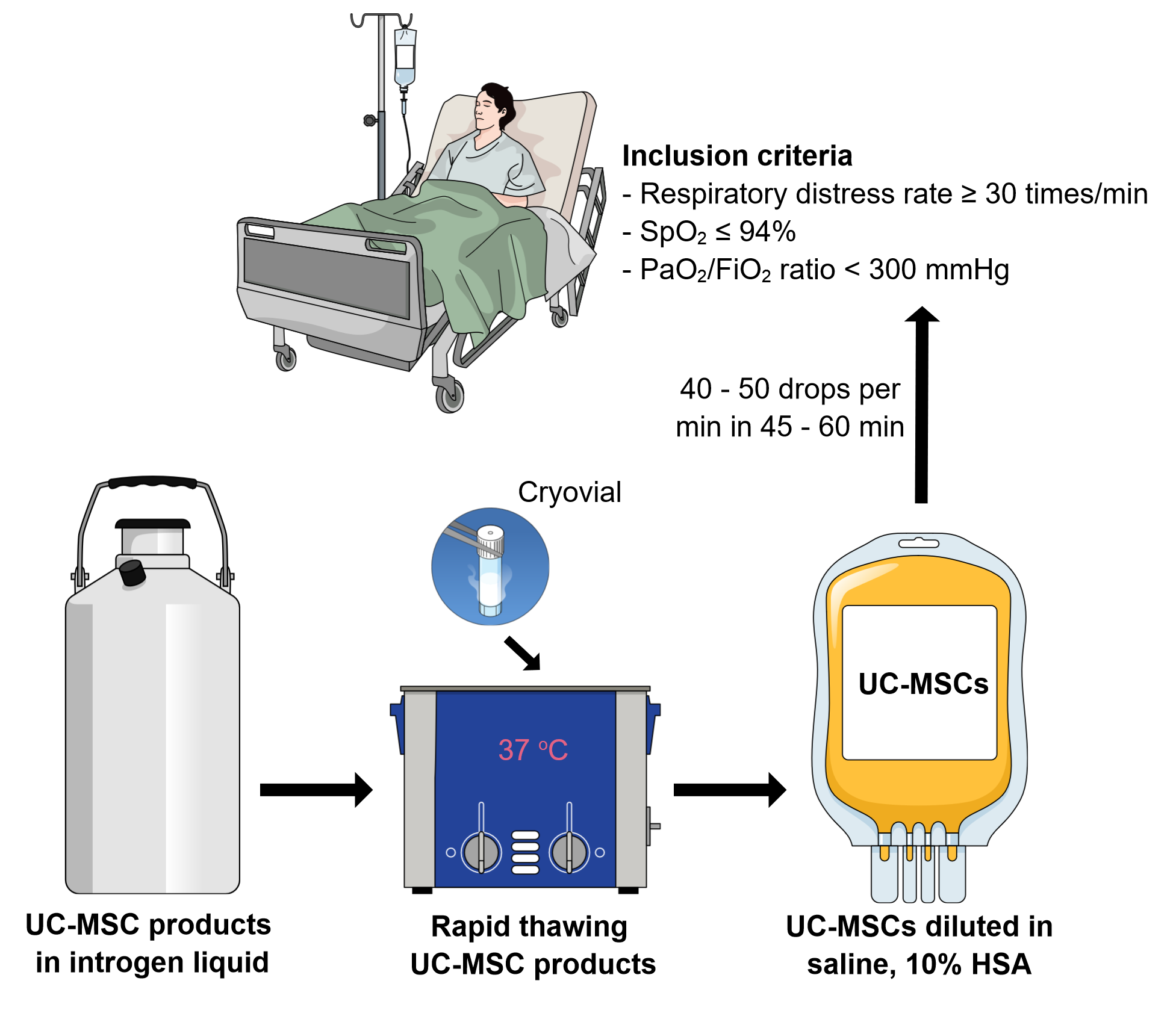
In 2020, we suggested that umbilical cord-derived mesenchymal stem cell (UC-MSC) transplantation can significantly improve COVID-19 symptoms based on evidential relations (10.4252/wjsc.v12.i8.721). One year later, this review aims to summarize and update the clinical evidence regarding UC-MSC usage in COVID-19 treatment. The publications on applications of UC-MSCs were searched in the PubMed, Web of Science, and Google scholar databases with the keywords “umbilical cord-derived mesenchymal stem cells” and “COVID-19”. All publications about clinical studies, from case reports to randomized controlled trials (RCTs), were used as clinical evidence in this review. The results showed 16 publications (4 randomized clinical trials, 3 pilot studies/phase 1 clinical trials, 3 case series reports, and 6 case reports) with a total of 395 COVID-19 patients that were provided with UC-MSC transplantation. All publications demonstrated that UC-MSC transplantation is safe, well tolerated, improved COVID-19 symptoms, and significantly decreased mortality. These findings support our suggestion for the usage of off-the-shelf UC-MSCs for COVID-19 as an adjuvant therapy.
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
