Orally Administered Fisetin Reduces the Symptoms of Acute Allergic Asthma in a Preclinical Mouse Model
DOI:
https://doi.org/10.15419/bmrat.v9i3.731Keywords:
Allergic asthma, natural products, ovalbumin, eosinophilic inflammation, TH2 responseAbstract
Background: Asthma is a very common, non-communicable, chronic respiratory disease, with a high incidence rate worldwide. Currently, no permanent therapeutic strategies are available for the treatment of asthma. Drugs, such as corticosteroids, β2-agonists, and anticholinergics, only temporarily reduce symptoms and have various adverse effects. In this study, we investigated the use of fisetin, a readily available natural product found in fruits, such as strawberries and apples, to treat acute asthma in a preclinical mouse model.
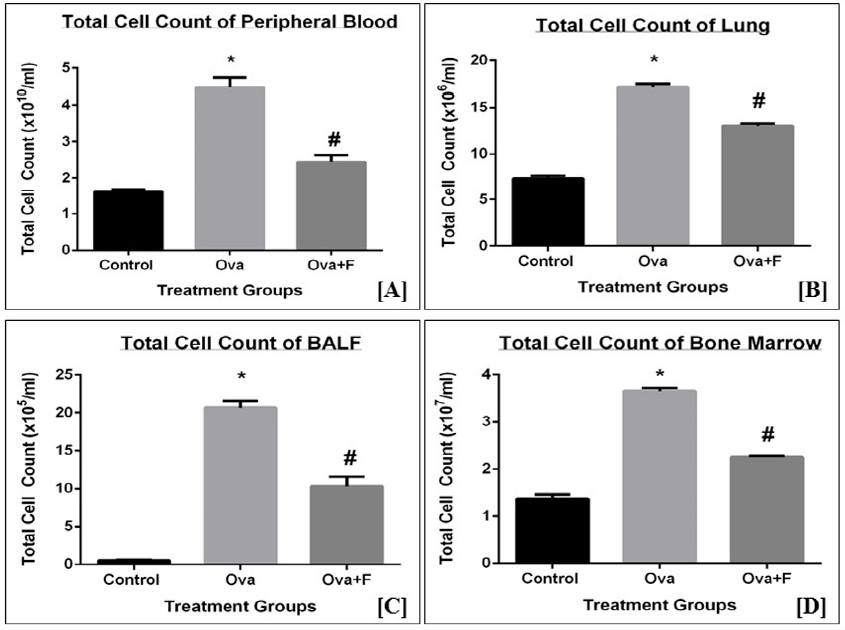
Methods: We induced asthma in BALB/c mice using intraperitoneal sensitization and repeated intratracheal challenges with ovalbumin (Ova), followed by oral administration of 0.6 mg/kg (MPK) aqueous fisetin (primary stock in DMSO) before each intratracheal challenge. The mice were subsequently evaluated with whole-body plethysmography, 24 hours after the last intratracheal challenge, to measure airway hyper-responsiveness (AHR). The mice were sacrificed, and their tissues removed to evaluate a range of disease parameters.
Results: In mice treated with fisetin, AHR was significantly reduced, as shown by plethysmography. Acute inflammation associated with asthma was also reduced, as determined by inhibition of cellular infiltration, serum IgE, and serum nitric oxide (NO), as well as a reduction in goblet cell hyperplasia. We also found that fisetin potentially acts by blocking the TH2 response, including pro-inflammatory signaling molecules such as NFκB, STAT6, HIF1α, iNOS, TNFα and IL13.
Conclusion: Our study showed that a low dose of fisetin (50 nM), administered orally in an aqueous solution, may offer a safe and economical therapeutic strategy for asthma.
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
