Assessment of the safety and effectiveness of the first sirolimus stent manufactured in Viet Nam
DOI:
https://doi.org/10.15419/bmrat.v9i7.751Keywords:
coronary artery disease, sirolimus-eluting stents, restenosis in stentsAbstract
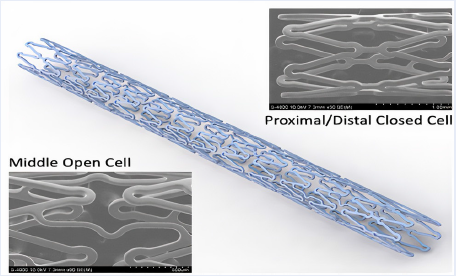
Objectives: To evaluate the effectiveness and safety of the first sirolimus-eluting stent made in Vietnam (Xplosion stent) on Vietnamese patients via the following outcomes: restenosis rate in the stent and at the two heads of stent, stent occlusion rate, and mortality rate due to myocardial infarction at 6 months and 12 months following stent placement.
Methods and Results: The prospective, open-label, non-randomized, longitudinal study enrolled 43 patients diagnosed with stable angina and de novo lesions with a 2.5 – 3.5 mm reference diameter and 24 mm in length. The patients received one stent per branch, and some received stents in multiple branches. All patients were scheduled for angiographic follow-up at 6 months, and if subjects consented, at 12 months. The subjects' mean age was 60.05 +/- 11.07 years, and many had cardiovascular risk factors, such as hypertension (83.72%), dyslipidemia (53.49%), and diabetes mellitus (25.58%). Stenting was performed on 50 lesions with a sirolimus drug-eluting stent (1.16 stent/patient on average). Of the patients, 92% had single-vessel coronary artery disease, and type B lesions (AHA) accounted for the majority (56%), with distribution on the LAD (42%), LCx (24%), and RCA (34%). The technical and procedural success rate of stent placement was 97.67%, with a very low complication rate (0%). The restenosis rate at 6 months was 0%. All patients were followed up to 12 months. Only two patients had recurrent chest pain and underwent coronary angiography; however, there was no in-stent restenosis and no need for revascularization. Therefore, the rate of chest pain recurrence after 12 months was 4.65% (2/43), and the rate of target vessel revascularization at 12 months was 0%. At 6- and 12-month follow-up, we observed no death due to unknown causes, no target-vessel myocardial infarction, and two clinically-driven re-angiographies with no need for revascularization. No additional events were reported beyond the 6-month follow-up. During the entire 12-month follow-up period, none of the patients experienced a definite or probable stent thrombosis.
Conclusion: The new sirolimus-eluting stents manufactured in Vietnam use a new technology transferred from the United States. They were placed successfully and showed a sustained favorable safety profile for up to 12 months. These findings suggest that these new stents could be used in many catheterization laboratories in Viet Nam.
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
