A review of fibroblast-like synoviocytes in the pathogenesis of Rheumatoid arthritis: Their activation and the inhibition of their apoptosis
DOI:
https://doi.org/10.15419/bmrat.v9i11.779Keywords:
Apoptosis, inflammation, matrix-metalloproteinase, pro-inflammatory cytokine, rheumatoid arthritis, synovial fibroblastsAbstract
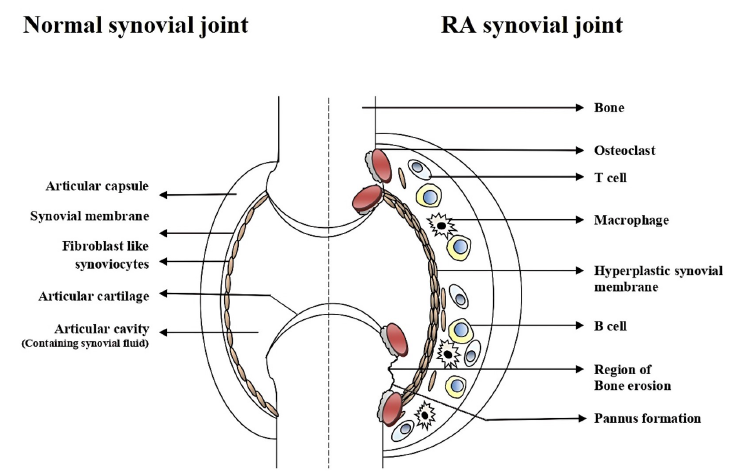
Rheumatoid Arthritis (RA) is a systemic, autoimmune, inflammatory disease characterized by synovial hyperplasia, inflammatory cell infiltration in the synovial tissues, and progressive destruction of cartilage and bones. This disease often leads to chronic disability. More recently, activation of synovial fibroblasts (SFs) has been linked to innate immune responses and several cellular signalingpathways that ultimately result in the aggressive and invasive stages of RA. SFs are the major sources of pro-inflammatory cytokines in RA synovium. They participate in maintaining the inflammatory state that leads to synovial hyperplasia and angiogenesis in the inflamed synovium. The altered apoptotic response of synovial and inflammatory cells has been connected to these alterations of inflamed synovium. RA synovial fibroblasts (RASFs) have the ability to inhibit several apoptotic proteins that cause their abnormal proliferation. This proliferation leads to synovial hyperplasia. Apoptotic pathway proteins have thus been identified as possible targets for modifying the pathophysiology of RA. This review summarizes current knowledge of SF activation and its roles in the inhibition of apoptosis in the synovium, which is involved in joint damage during the effector phase of RA development.
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
