Effects of Remdesivir and Favipiravir on Covid-19 Clinical Outcomes : A Systematic Review and Meta-Analysis
DOI:
https://doi.org/10.15419/bmrat.v10i5.811Keywords:
Remdesivir, Favipiravir, Covid-19, Mortality, ICU admissionAbstract
Introduction: After nearly two years, there is still no proven treatment for infection with severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)?the virus that causes Covid-19. Currently, the two most widely known drugs for treating Covid-19 are remdesivir and favipiravir. Therefore, this study aimed to evaluate the effects of remdesivir and favipiravir on Covid-19 clinical outcomes.
Methods: A systematic review of the literature on the PubMed and Scopus databases was undertaken to identify studies that have examined the effects of remdesivir and favipiravir on Covid-19 outcomes. To weighted group mean differences for within- and between-group comparisons, odds ratio effect sizes, and random-effects models were used. Subgroup analyses were also conducted to determine the effects of potential sources of heterogeneity, which was assessed using the I-squared (I2) test.
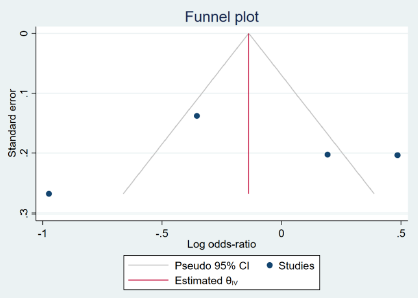
Results: Twenty-eight studies with a total of 10,871 adult participants were included in the analysis. According to pooled analysis results, there was no statistically significant difference between the remdesivir/favipiravir and control groups in terms of mortality, intensive care unit admissions, or adverse effects (p > 0.05). Mean hospitalization duration was significantly different for those receiving remdesivir (0.1-day increase) and favipiravir (0.06-day decrease), but these findings included significant levels of publication bias. Treatment duration was found to be a significant source of heterogeneity in the mortality results.
Conclusion: Remdesivir and favipiravir have no effect on mortality, intensive care unit admissions, or duration of hospitalization for Covid-19 patients.
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
