Assessment of siRNA as a therapeutic molecule in Transient Receptor Potential Channel 5 gene silencing: a computational approach
DOI:
https://doi.org/10.15419/bmrat.v5i1.405Keywords:
Albuminuria, gene silencing, RNAi, siRNA, TRPC5Abstract
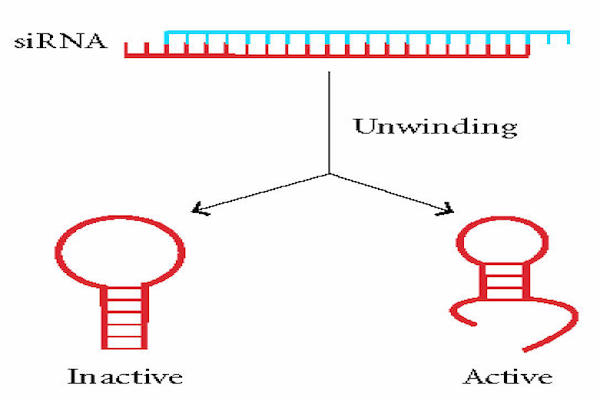
Background: Ion channels play a crucial role in Glomerular filter damage that contributes to albuminuria. Transient receptor potential channel 5 (TRPC5) gene mediating such damage, demand for its target specific inhibition by RNA interference mechanism. Designing and selecting potential siRNA for TRPC5 gene silencing by computational analysis.
Materials & Methods: The mRNA sequence was retrieved from NCBI (National Center for Biotechnology Information). siRNA sequences were designed specifically from target genes using InvivoGen siRNA wizard software. Thermodynamic RNA-RNA interactions were used to evaluate the gene silencing efficiency by minimum free energy of hybridization; the hybridization structures were also obtained using BIBISERV2-RNAHybrid.
Results: The minimum free energy of hybridization of the three designed siRNAs (siRNA1, siRNA2 and siRNA3) were as follows: -28.2 kcal/mol, -24.1 kcal/mol, and-25.6 kcal/mol. Their corresponding GC content were 47.62%, 52.38% and 47.62%, respectively. Thus, siRNA1 had the least minimum free energy of hybridization (i.e. -28.2 kcal/mol) with low GC content (47.62%), and high linearity with minimal h-b index and loop structure.
Conclusion: RNAi therapy can provide a new platform for efficient and targeted therapeutics. Further in vivo investigations are necessary to further validate their efficacy.
References
Anavekar, N. S., Gans, D. J., Berl, T., Rohde, R. D., Cooper, W., Bhaumik, A., . . . Pfeffer, M. A. (2004). Predictors of cardiovascular events in patients with type 2 diabetic nephropathy and hypertension: A case for albuminuria. Kidney International. Supplement, 92(92), S50–S55. https://doi.org/10.1111/j.1523-1755.2004.09213.x PMID:15485418
Bernstein, E., Caudy, A. A., Hammond, S. M., & Hannon, G. J. (2001). Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature, 409(6818), 363–366. https://doi.org/10.1038/35053110 PMID:11201747
Berrut, G., Bouhanick, B., Fabbri, P., Guilloteau, G., Bled, F., Le Jeune, J. J., . . . Marre, M. (1997). Microalbuminuria as a predictor of a drop in glomerular filtration rate in subjects with non-insulin-dependent diabetes mellitus and hypertension. Clinical Nephrology, 48(2), 92–97. PMID:9285145
Bret SE, Harris S. Soifer, Chauncey Bowers, and John J. Rossi.(2005). siRNA target site secondary structure predictions using local stable substructures. Nucleic Acid Res, 33(3), e30. https://doi.org/10.1093/nar/gni026
Brummelkamp, T. R., Bernards, R., & Agami, R. (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science, 296(5567), 550–553. https://doi.org/10.1126/science.1068999 PMID:11910072
Czech, M. P., Aouadi, M., & Tesz, G. J. (2011). RNAi-based therapeutic strategies for metabolic disease. Nature Reviews. Endocrinology, 7(8), 473–484. https://doi.org/10.1038/nrendo.2011.57 PMID:21502982
Elbashir, S. M., Lendeckel, W., & Tuschl, T. (2001). RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes & Development, 15(2), 188–200. https://doi.org/10.1101/gad.862301 PMID:11157775
Farquhar, M. G. (2006). The glomerular basement membrane: Not gone, just forgotten. The Journal of Clinical Investigation, 116(8), 2090–2093. https://doi.org/10.1172/JCI29488 PMID:16886057
Faul, C., Donnelly, M., Merscher-Gomez, S., Chang, Y. H., Franz, S., Delfgaauw, J., . . . Mundel, P. (2008). The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nature Medicine, 14(9), 931–938. https://doi.org/10.1038/nm.1857 PMID:18724379
Gerstein, H. C., Mann, J. F., Yi, Q., Zinman, B., Dinneen, S. F., Hoogwerf, B., . . . Yusuf, S., & the HOPE Study Investigators. (2001). Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. Journal of the American Medical Association, 286(4), 421–426. https://doi.org/10.1001/jama.286.4.421 PMID:11466120
Hammond, S. M., Bernstein, E., Beach, D., & Hannon, G. J. (2000). An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature, 404(6775), 293–296. https://doi.org/10.1038/35005107 PMID:10749213
Haraldsson, B., Nyström, J., & Deen, W. M. (2008). Properties of the glomerular barrier and mechanisms of proteinuria. Physiological Reviews, 88(2), 451–487. https://doi.org/10.1152/physrev.00055.2006 PMID:18391170
Jackson, A. L., & Linsley, P. S. (2010). Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nature Reviews. Drug Discovery, 9(1), 57–67. https://doi.org/10.1038/nrd3010 PMID:20043028
Keane, W. F., Brenner, B. M., de Zeeuw, D., Grunfeld, J. P., McGill, J., Mitch, W. E., . . . Toto, R., & the RENAAL Study Investigators. (2003). The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: The RENAAL study. Kidney International, 63(4), 1499–1507. https://doi.org/10.1046/j.1523-1755.2003.00885.x PMID:12631367
Ma, H., Togawa, A., Soda, K., Zhang, J., Lee, S., Ma, M., . . . Ishibe, S. (2010). Inhibition of podocyte FAK protects against proteinuria and foot process effacement. Journal of the American Society of Nephrology, 21(7), 1145–1156. https://doi.org/10.1681/ASN.2009090991 PMID:20522532
Ma, Y., Chan, C. Y., & He, M. L. (2007). RNA interference and antiviral therapy. World Journal of Gastroenterology, 13(39), 5169–5179. https://doi.org/10.3748/wjg.v13.i39.5169 PMID:17876887
Mathews, D. H. (2005). Predicting a set of minimal free energy RNA secondary structures common to two sequences. Bioinformatics (Oxford, England), 21(10), 2246–2253. https://doi.org/10.1093/bioinformatics/bti349 PMID:15731207
Mogensen, C. E. (1984). Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. The New England Journal of Medicine, 310(6), 356–360. https://doi.org/10.1056/NEJM198402093100605 PMID:6690964
Montell, C. (2005). TRP channels in Drosophila photoreceptor cells. The Journal of Physiology, 567(Pt 1), 45–51. https://doi.org/10.1113/jphysiol.2005.092551 PMID:15961416
Ramsey, I. S., Delling, M., & Clapham, D. E. (2006). An introduction to TRP channels. Annual Review of Physiology, 68(1), 619–647. https://doi.org/10.1146/annurev.physiol.68.040204.100431 PMID:16460286
Rehmsmeier, M., Steffen, P., Hochsmann, M., & Giegerich, R. (2004). Fast and effective prediction of microRNA/target duplexes. RNA (New York, N.Y.), 10(10), 1507–1517. https://doi.org/10.1261/rna.5248604 PMID:15383676
Reynolds, A., Leake, D., Boese, Q., Scaringe, S., Marshall, W. S., & Khvorova, A. (2004). Rational siRNA design for RNA interference. Nature Biotechnology, 22(3), 326–330. https://doi.org/10.1038/nbt936 PMID:14758366
Riccio, A., Li, Y., Moon, J., Kim, K. S., Smith, K. S., Rudolph, U., . . . Clapham, D. E. (2009). Essential role for TRPC5 in amygdala function and fear-related behavior. Cell, 137(4), 761–772. https://doi.org/10.1016/j.cell.2009.03.039 PMID:19450521
Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K., & Tuschl, T. (2001). Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411(6836), 494–498. https://doi.org/10.1038/35078107 PMID:11373684Thomas schaldecker, Sookyung Kim, Constantine Tarabanis, Dequan Tian, Samy Hakroush, Philip Castonguay, Wooin Ahn, Hanna Wallentin, Hans Heid, Corey R. Hopkins, Craig W. Lindsley, Antonio Riccio, Lisa Buvall,Astrid Weins, Anna Greka.(2013). Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest,123(12), 5298–5309.
Tian, D., Jacobo, S. M., Billing, D., Rozkalne, A., Gage, S. D., Anagnostou, T., . . . Greka, A. (2010). Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Science Signaling, 3(145), ra77. https://doi.org/10.1126/scisignal.2001200 PMID:20978238
Togawa, A., Miyoshi, J., Ishizaki, H., Tanaka, M., Takakura, A., Nishioka, H., . . . Takai, Y. (1999). Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene, 18(39), 5373–5380. https://doi.org/10.1038/sj.onc.1202921 PMID:10498891Tuschl, T., & Borkhardt, A. (2002). Small interfering RNAs: A revolutionary tool for the analysis of gene function and gene therapy. Molecular Interventions, 2(3), 158–167. https://doi.org/10.1124/mi.2.3.158 PMID:14993376
Xu, S. Z., Sukumar, P., Zeng, F., Li, J., Jairaman, A., English, A., . . . Beech, D. J. (2008). TRPC channel activation by extracellular thioredoxin. Nature, 451(7174), 69–72. https://doi.org/10.1038/nature06414 PMID:18172497
Yanagida-Asanuma, E., Asanuma, K., Kim, K., Donnelly, M., Young Choi, H., Hyung Chang, J., . . . Mundel, P. (2007). Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. American Journal of Pathology, 171(2), 415–427. https://doi.org/10.2353/ajpath.2007.070075 PMID:17569780Zamore, P. D., Tuschl, T., Sharp, P. A., & Bartel, D. P. (2000). RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell, 101(1), 25–33. https://doi.org/10.1016/S0092-8674(00)80620-0 PMID:10778853
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
