Survival analysis of liver cirrhosis patients after transplantation using accelerated failure time models
DOI:
https://doi.org/10.15419/bmrat.v5i11.495Keywords:
Accelerated Failure Time models, Liver, TransplantationAbstract
Introduction: Liver transplantation is known as the only treatment for advanced liver cirrhosis. Considering the importance of identifying the factors affecting the survival of cirrhosis patients after transplantation in order to improve the health of these patients and increase their longevity, this study was conducted to fit the best accelerated failure time model for survival analysis of cirrhosis patients.
Methods: This descriptive-analytical study was conducted by collecting the information about 563 patients with liver cirrhosis who underwent liver transplantation in Imam Khomeini Hospital during 2002-2013 and were followed up for at least 5 years. The data were analyzed using Chisquare test, ANOVA, and Kaplan-Meier non-parametric method as well as exponential Accelerated Failure Time, Weibull, Log-Normal, and Log-Logistic survival models.
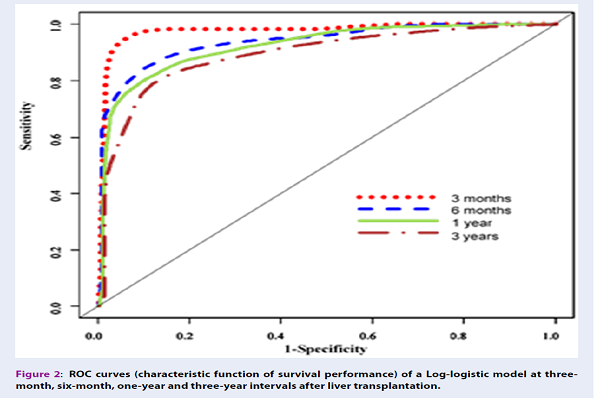
Results: During the study, 92 (16.3%) of the 563 patients under study died and 165 (29.3%) of them suffered liver transplant rejection. The one-year, three-year, and five-year survival of the patients after transplantation was 0.804, 0.653, and 0.420, respectively. Among the fitted Accelerated Failure Time models, the fitted log-logistic model was the most effective (P-value < 0.001). The effective variables in the Multiple regression log-logistic model included bilirubin (P-value < 0.001), INR (P-value < 0.001), creatinine (P-value < 0.001), and white blood cell (P-value = 0.011) logarithms.
Conclusion: Regarding the results of the study, bilirubin, INR, creatinine, and white blood cell logarithmic variables were effective in the survival analysis of the patients after liver transplantation. The survival of these patients can be enhanced through necessary care to maximally control these variables.
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
