Diabetes mellitus type 2 reduces the viability, proliferation, and angiogenic marker of adipose-derived stem cells cultured in low-glucose anti-oxidant-serum supplemented medium
DOI:
https://doi.org/10.15419/bmrat.v6i3.530Keywords:
adipose-derived stem cells, CFU-F, cell viability, diabetic, endoglinAbstract
Introduction: Hyperglycemia in diabetic patients induces elevated pro-inflammatory cytokine production, resulting in cellular damage, which may affect the regenerative function of mesenchymal stem cells (MSCs), such as adipose-derived stem cells (ADSCs). Identifying the effect of diabetes on ADSCs and optimization of culture conditions is therefore an important starting point for the application of autologous stem cells to improve clinicial outcomes. The aim of this study was to investigate the effect of diabetes on ADSCs that cultured in low-glucose anti-oxidant-serum supplemented medium.
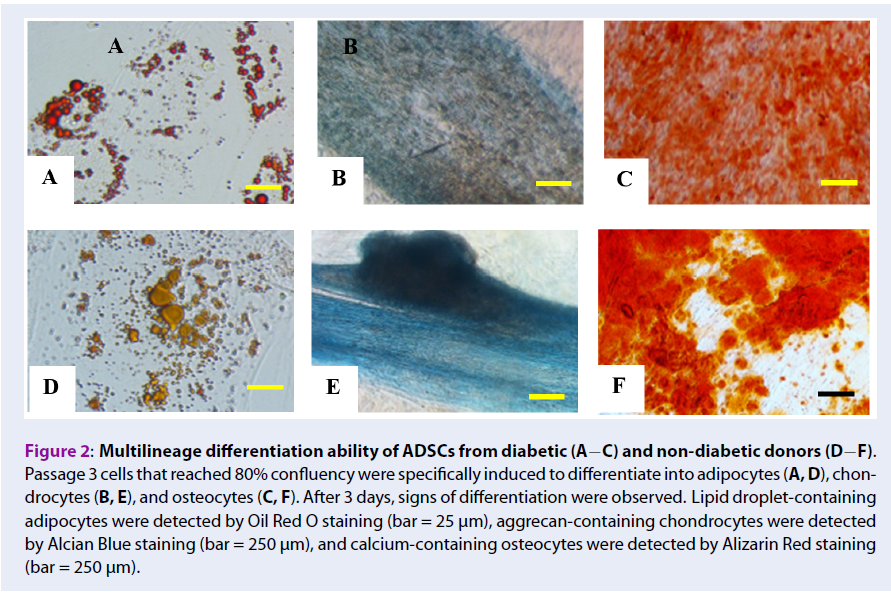
Methods: In this study, freshly isolated stromal vascular fraction (SVF) and expanded ADSCs were compared between diabetic and non-diabetic donors. SVF were isolated from the abdominal fat, and total viable cells and viability were estimated. Fresh SVF were cultured in low-glucose (100 mg/dL) culture medium supplemented with an anti-oxidant and fetal bovine serum (complete culture medium) at a low density for 14 days for the colony formation unit-fibroblast (CFU-F) assay. The remaining SVF were expanded to obtain ADSCs in the complete culture medium, which were evaluated based on MSCs surface marker expression and three lineage differentiation potential. Diabetic and non-diabetic ADSCs were compared with respect to population doubling time and viability after serial passage.
Results: Total viable counts (0.97 +/- 0.39 x 109 cells/10 mL of adipose tissue, 0.56 +/- 0.39 x 109 cells/10 mL of adipose tissue, p=0.02, independent t-test), but not viability (98.63 +/- 1.12%, 98.20 +/- 1.21%, p= 0.38, independent t-test), were significantly higher for SVF cells from adipose tissues of non-diabetic donors than diabetic donors. Fewer CFU-F were obtained from cultured diabetic SVF than from non-diabetic SVF. Diabetic and non-diabetic ADSCs had similar differentiation potency and CD73 (99.44 +/- 0.34%, 97.15 +/- 5.37%, p= 0.21, Mann-Whitney U test) and CD90 (97.30 +/- 2.86%, 95.06 +/- 6.32%, p= 0.90, Mann- Whitney U test) expression, but significantly fewer diabetic ADSCs expressed CD105 or endoglin, a marker for angiogenesis (89.91 +/- 7.14%, 57.90 +/- 21.36% for non-diabetic and diabetic groups, p< 0.001, Mann-Whitney U test). Diabetic ADSCs tended to exhibit slower proliferation (4.43 +/- 2.70 days, 3.04 +/- 0.55 days, p= 0.27 in passage 2 (P2); 3.95 +/- 1.55 days, 2.96 +/- 0.91 days, p= 0.21 in P3, independent t-test) and lower viability than those of non-diabetic ADSCs (77.65 +/- 10.61%, 87.13 +/- 10.06%, p= 0.25 in P2; 82.70 +/- 8.07%, 91.15 +/- 3.77%, p= 0.04 in P3, independent t-test). Culture in low-glucose anti-oxidant-serum supplemented medium did not improve CD105 expression (65.14 +/- 5.86%, 71.06 +/- 10.27%, 64.05 +/- 10.04%, p= 0.70, for P1, P2, and P3, respectively, repeated measure ANOVA) and cell proliferation (p= 0.50 for P2 vs. P3, paired t-test) of diabetic ADSCs.
Conclusions: Overall, diabetes reduced CD105 expression and ADSCs proliferation, suggesting that the angiogenic potency of diabetic ADSCs is reduced. The diabetic ADSCs in this study were also more prone to cell death caused by handling technique compared to non-diabetic ADSCs. Therefore, more advanced culture techniques should be applied to expand ADSCs from diabetic patients to achieve expected clinical outcomes.
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
