Outcome analysis of acute myeloid leukemia patients treated with high dose daunorubicin
DOI:
https://doi.org/10.15419/bmrat.v6i9.562Keywords:
Acute Myeloid Leukemia, Induced Therapy, Disease Free Survival, Overall Survival, Cytarabine, DaunorubicinAbstract
Abstract: Acute Myeloid Leukemia (AML) is a malignant hematopoietic disease caused by the presence of a malignant clone in the bone marrow. The classic AML treatment includes a combination of an Anthracycline and Cytarabine. This study aimed to evaluate the effect of high doses of Daunorubicin on patients' outcome.
Methods: During the study period, 16 AML patients received induction therapy with Cytarabine (100 mg/m2/d) for 7 days and Daunorubicin (90 mg/m2/d) for 3 days. Outcome analysis was performed to evaluate the overall survival (OS) and disease-free survival (DFS) during 2 years of study.
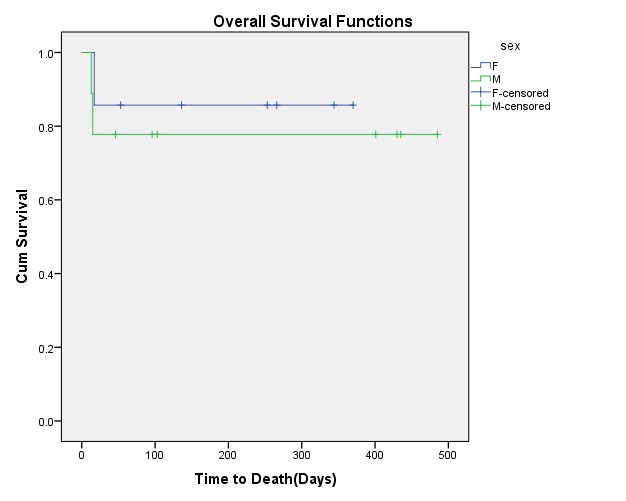
Results: The mean age of patients was 38+/-12.38 years, with the age range between 16 and 54 years old. Seven patients (43.8%) were females, and 9 cases (56.3%) were males. OS was 81.3%, with a mean of 396.88 days. (95% CI: 306.99-486.77). DFS was 83.3%, with a mean of 383.57 days (95% CI: 299.88-467.26). The log-rank test showed a significant difference in DFS of AML sub-types, as M1 subtypes had lower DFS (P log-rank= 0.013). Although M1 subtypes had a lower OS, there was no significant difference in OS between subgroups (P log-rank= 0.067).
Conclusion: Although disease-free survival was improved by increasing the dose of daunorubicin, there was no difference in the overall survival between the AML subgroups and sexes.
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
