Comparison of injection of midazolam-fentanyl with pethidine in management of pain induced by extracorporeal shock wave lithitripsy: A randomized clinical trial
DOI:
https://doi.org/10.15419/bmrat.v7i4.599Keywords:
Pain, Extracorporeal shock wave lithotripsy, Pethidine, MidazolamAbstract
Introduction: Urinary stones are the third most common disease of the genitourinary tract after urinary tract infections and prostate diseases. One of the ways to remove a urinary stone is extracorporeal shock wave lithitripsy, which crushes the stones for easier removal, and it is often used to reduce pain, reduce anxiety, and stabilize the patient. In this regard, the use of effective analgesics with less serious side effects seems reasonable.
Methods: This randomized clinical trial study was performed with 90 patients who were divided into two groups according to a random number table. The first group received pethidine, and the second group received midazolam and fentanyl (midazolam-fentanyl). The type of medication used and demographic information were recorded, and the patients’ pain was assessed by a visual analog pain scale at 15, 30, 45, and 60 minutes.
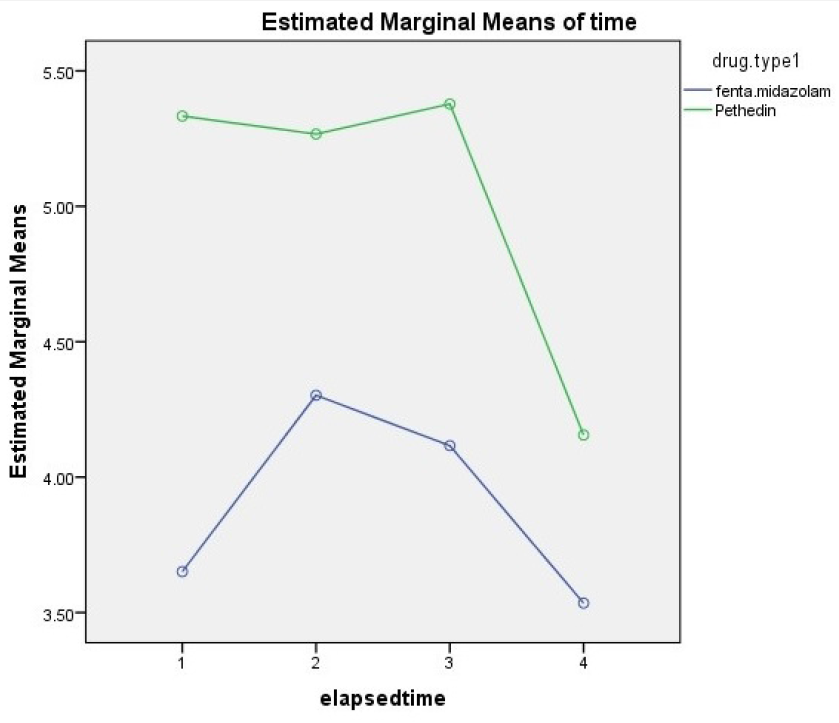
Results: Of the subjects, 59 (65.5%) were male and 30 (33.5%) were female. The mean age of the patients was 38.4 ± 13.5 years. The level of pain at 15 minutes was 3.71 ± 2.4 in the midazolam-fentanyl group and 5.33 ± 2.9 in the pethidine group. At 45 minutes, the pain level was 4.2 ± 3.1 in the midazolam-fentanyl group and 5.26 ± 2.72 in the pethidine group. The differences between groups was significant at 15 and 45 minutes. At 30 and 60 minutes, the pain was lower in the midazolam-fentanyl group than in the pethidine group, but these differences were not statistically significant. There was no significant difference between the two groups with respect to the incidence of nausea and vomiting, restlessness, and anxiety.
Conclusion: This study showed that the pain reported by patients using fentanyl-midazolam was lower than the pain reported by patients on pethidine, and the pain decreased with time in both groups. Therefore, if there is no other indication for the use of drugs, the combination of fentanyl-midazolam will have a better effect on pain and should be used.
Trial registration: Current Controlled Trials IRCT2016051427893N1.
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
