Docking studies, synthesis, characterization, and cytotoxicity activity of new bis-chalcones derivatives
DOI:
https://doi.org/10.15419/bmrat.v8i4.668Keywords:
bis-chalcone, cyclo ketone, breast cancer, tamoxifen, terephthalaldehydeAbstract
Introduction: Breast cancer is women's most prevalent cancer type. The development of chemotherapy drug resistance and adverse effects is a significant barrier to breast cancer treatment. Recently, the focus of drug discovery has increased toward a valuable structure known as chalcones due to their extensive bioactivity in cancer treatment.
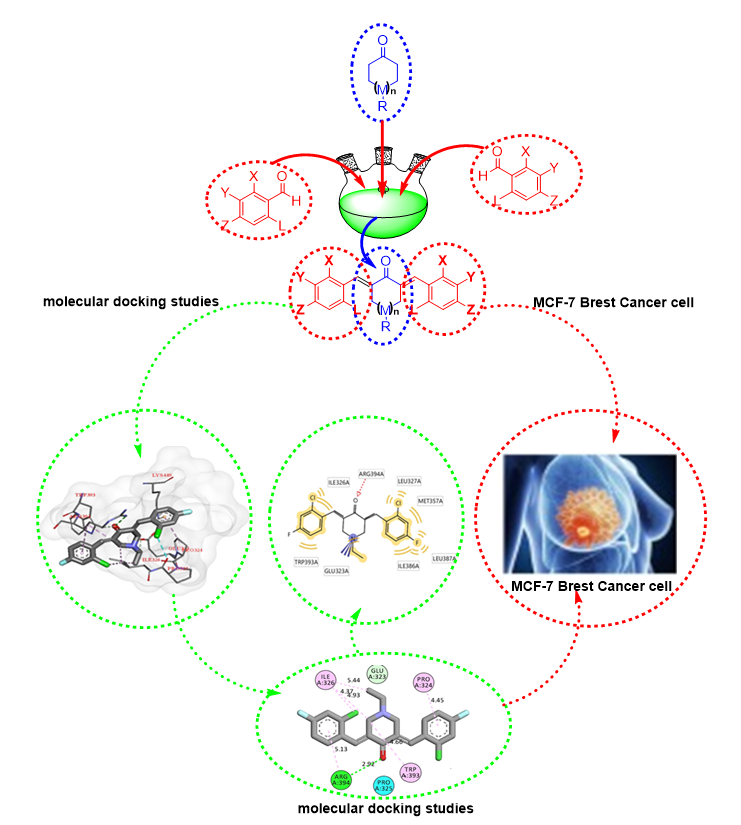
Methods: The molecular docking studies were conducted using the Discovery Studio (DSv4.5) and MG. Tools installed in Window 10. The cancer receptor (3ERT) was downloaded from the protein data bank (PDB). All new compounds were characterized by IR, 1H-NMR, 13C-NMR, 2D-NMR, and CHN elemental analysis.
Results: A bis-chalcones (1-13) were prepared by the reaction of terephthalaldehyde with 3-acetyl-2,5-dichlorothiophene or 3-acetyl- 5-chlorothiophene and reaction cyclo ketone derivatives with phenyl aldehyde derivatives in good yields. All the synthesized bis chalcone derivatives have been characterized using ATR-FTIR, NMR (1D and 2D). The cytotoxicity activity of all these chalcone compounds was investigated against breast cancer cell line (MCF-7). The results showed that compound 6 showed more potent activity in inhibiting growth on both types of MCF-7 IC50 (4.4 ± 0.10 µM) compared to reference tamoxifen IC50 (17.9 ± 1.2 µM).
Conclusion: The cytotoxic activity of all the synthesized compounds was evaluated against MCF-7 breast cancer cell lines. Compounds 5 and 6 were found to have IC50 values of about 20 µM. These compounds are good candidates to be selected for further studies to develop anticancer drugs. The structure-activity relationships study showed that bis-chalcone compounds with substituted chlorine and flurine atom at the ortho-chlorine and para-flurine position of their aromatic rings exhibited greater cytotoxic activity against MCF-7 cell line compared to other compounds. Moreover, in the cytotoxic activity test towards MCF-7 breast cancer cells, bis-chalcones derivatives from cyclohexane, 1-ethylpiperidin-4-one were found to be more potent than bis-chalcone derivatives from acetone, cyclopentanone, 4-(tert-butyl)cyclohexane-1-one, and terephthalaldehyde.
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
