CAR-T-Cell Therapy: Present Progress and Future strategies
DOI:
https://doi.org/10.15419/bmrat.v9i2.726Keywords:
CAR-T, Immunotherapy, Malignancies, T-cell receptor, Tumor antigensAbstract
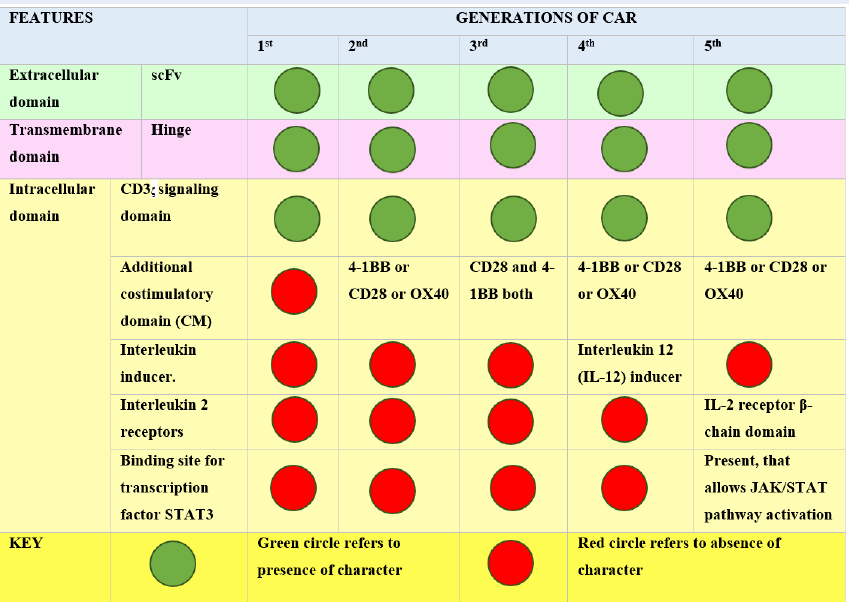
Chimeric antigen receptor (CAR) T-cell therapy is a type of immunotherapy that uses the patient's immune system. It creates cancer-killing T cells through genetic modification that targets tumor antigens. CAR consists of three fundamental units, the extracellular, transmembrane, and intracellular domains. CARs are rapidly evolving with progress in the field of immunotherapy starting from first-generation CARs to next-generation CARs. Different cancer types, including B-cell malignancies, are being treated by CAR-T therapy. The FDA has approved two CAR-T therapies, namely, tisagenlecleucel and axicabtagene ciloleucel. The recently approved CAR-T products are Lisocabtagene maraleucel and Idecabtagene vicleucel. Despite the success of CAR-T therapy, several limitations, including cytokine release syndrome and neurotoxicity, need to be overcome. In the present review, we have provided an overview of CAR generations, their applications, potential limitations, and possible solutions for improving CAR-T therapy for a variety of tumor types.
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
