Levofloxacin-based sequential therapy versus classic triple therapy in Helicobacter pylori eradication: A randomized clinical trial
DOI:
https://doi.org/10.15419/bmrat.v4i11.384Keywords:
Amoxicillin, Clarithromycin, H. pylori, Helicobacter pylori, Levofloxacin, Omeprazole, Randomized clinical trial, Sequential, MedicineAbstract
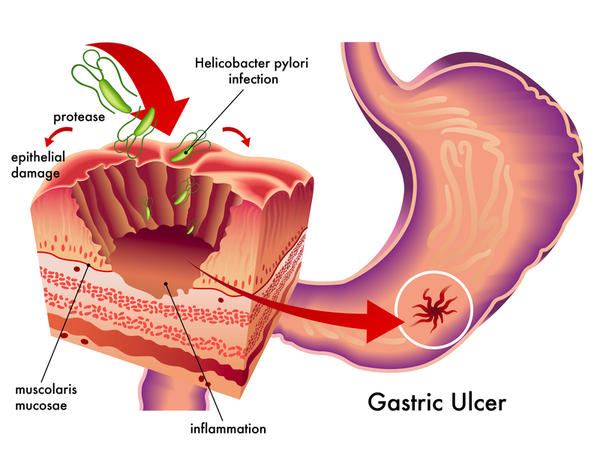
Introduction: During the past two decades, eradication rates with triple therapy for Helicobacter (H.) pylori eradication have decreased. The decline of effectiveness of the triple therapy have led to investigations to achieve more effective and safe therapeutic strategies. Sequential, concomitant and hybrid therapeutic regimens are new therapies that have been introduced over the past two decades. The purpose of this study was to compare levofloxacin-based sequential therapy versus classic triple therapy in H. pylori eradication in a randomized clinical trial.
Methods: All eligible cases were divided into two groups using a randomized block method. The first group (classic group) was treated with triple therapy; patients received omeprazole, amoxicillin and clarithromycin for 14 days. The second group received the levofloxacin-based sequential (lev-seq) regimen; the patients were treated with omeprazole and amoxicillin for the first 7 days and then with omeprazole, levofloxacin and metronidazole for the latter 7 days. In this clinical trial, 200 patients with H. pylori infection were enrolled. Ultimately, 192 patients completed the study. Of these, 95 patients (46 males and 49 females) were treated with triple therapy (classic group) and 97 patients (46 males and 51 females) were treated with the lev-seq regimen. The average age in the classic group and lev-seq groups were 41.4±12.4 years and 40.2±11.8 years, respectively.
Results: The levofloxacin based sequential regimen was more effective than triple therapy regimen. It is recommended that the lev-seq regimen be used as the first-line therapy, especially in areas with high levels of resistance to clarithromycin (over 20%).
Conclusion: Further studies with large numbers of samples are required to be conducted in different parts of the country for further evaluation of efficacy of this regimen.
References
<li class="show">Chey, W. D., & Wong, B. C. (2007). American College of Gastroenterology guideline on the management of Helicobacter pylori infection. The American Journal of Gastroenterology, 102(8), 1808–1825. <a href="https://doi.org/10.1111/j.1572-0241.2007.01393.x">https://doi.org/10.1111/j.1572-0241.2007.01393.x</a></li>
<li class="show">Park, J. Y., Dunbar, K. B., Mitui, M., Arnold, C. A., Lam-Himlin, D. M., Valasek, M. A., . . . Doern, C. D. (2016). Helicobacter pylori Clarithromycin resistance and treatment failure are common in the USA. Digestive Diseases and Sciences, 61(8), 2373–2380. <a href="https://doi.org/10.1007/s10620-016-4091-8 ">https://doi.org/10.1007/s10620-016-4091-8 </a></li>
<li class="show">Malfertheiner, P., Megraud, F., O’Morain, C. A., . . .. (2017). European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut, 66, 6–30. <a href="https://doi.org/10.1136/gutjnl-2016-312288">https://doi.org/10.1136/gutjnl-2016-312288</a></li>
<li class="show">De Francesco, V., Giorgio, F., Hassan, C., . . .. (2010). Worldwide H. pylori antibiotic resistance: A systematic review. Journal of Gastrointestinal and Liver Diseases; JGLD, 19, 409–414.</li>
<li class="show">Zullo, A., Rinaldi, V., Winn, S., Meddi., Lionetti., Hassan., . . . Attili. (2000). A new highly effective short-term Therapy schedule for Helicobacter pylori eradication. Alimentary Pharmacology & Therapeutics, 14(6), 715–718. <a href="https://doi.org/10.1046/j.1365-2036.2000.00766.x">https://doi.org/10.1046/j.1365-2036.2000.00766.x</a></li>
<li class="show">Vakil, N., & Vaira, D. (2008). Sequential therapy for Helicobacter pylori: Time to Consider making the switch? Journal of the American Medical Association, 300(11), 1346–1347. <a href="https://doi.org/10.1001/jama.300.11.1346">https://doi.org/10.1001/jama.300.11.1346</a></li>
<li class="show">Vaira, D., Zullo, A., Vakil, N., Gatta, L., Ricci, C., Perna, F., . . . Morini, S. (2007). Sequential therapy versus standard Triple-drug therapy for Helicobacter pylori eradication: A randomized Trial. Annals of Internal Medicine, 146(8), 556–563. <a href="https://doi.org/10.7326/0003-4819-146-8-200704170-00006">https://doi.org/10.7326/0003-4819-146-8-200704170-00006</a></li>
<li class="show">Molina-Infante, J., Perez-Gallardo, B., Fernandez-Bermejo, M., . . .. (2010). Clinical trial: Clarithromycin vs. levofloxacin in first-line triple and sequential regimens for Helicobacter pylori eradication. Alimentary Pharmacology & Therapeutics, 31, 1077–1084.</li>
<li class="show">Romano, M., Cuomo, A., Gravina, A. G., Miranda, A., Lovene, M. R., Tiso, A., . . .. (2010). Empirical Levofloxacin-containing versus Clarithromycin-containing sequential therapy for Helicobacter pylori eradication: A randomised trial. Gut, 59(11), 1465–1470. <a href="https://doi.org/10.1136/gut.2010.215350">https://doi.org/10.1136/gut.2010.215350</a></li>
<li class="show">Azadbakht S, Moradniani |M, Mirbeik Sabzevari Z, Hassani M and Tarrahi M J. (2017). The Prevalence of Intestinal Metaplasia in Dyspeptic Patients. [IJBR]. International Journal of Advanced Biotechnology and Research, 8(3), 2156–2165.</li>
<li class="show">Khademi, F., Poursina, F., Hosseini, E., Akbari, M., & Ghasemian Safaei, H. (2015). Helicobacter pylori in Iran: A systematic review on the antibiotic resistance. Iranian Journal of Basic Medical Sciences., 18, 2–7.</li>
<li class="show">Jafri, N. S., Hornung, C. A., & Howden, C. W. (2008). Meta-analysis: Sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to Treatment. Annals of Internal Medicine, 148(12), 923–931. <a href="https://doi.org/10.7326/0003-4819-148-12-200806170-00226">https://doi.org/10.7326/0003-4819-148-12-200806170-00226</a></li>
<li class="show">Gatta, L., Vakil, N., Vaira, D., & Scarpignato, C. (2013). Global eradication rates for Helicobacter pylori infection: Systematic review and meta-analysis of sequential therapy. BMJ (Clinical Research Ed.), 347(aug07 1), f4587. <a href="https://doi.org/10.1136/bmj.f4587">https://doi.org/10.1136/bmj.f4587</a></li>
<li class="show">Moradniani, M., Mirbeik-Sabzevari, Z., Bahmani, M., Azadbakht, S., & Jaferian, S. (2017). Comparison of 7 - day concomitant therapy regimen versus classic triple therapy regimen in Helicobacter pylori eradication: A randomized clinical trial. Int J Pharm Sci Res, 8(8), 3568–3573.</li>
<li class="show">Fakheri, H., Bari, Z., Aarabi, M., & Malekzadeh, R. (2014). Helicobacter pylori eradication in West Asia: A review. World Journal of Gastroenterology, 20(30), 10355–10367. <a href="https://doi.org/10.3748/wjg.v20.i30.10355">https://doi.org/10.3748/wjg.v20.i30.10355</a></li>
<li class="show">Moradniani, M., Azadbakht, S., & Jaferian, S. (2017). levofloxacin versus clarithromycin sequential therapy. Gastroenterology and Hepatology from Bed To Bench, •••, 10.</li>
<li class="show">Ozdil, K., Calhan, T., Sahin, A., Şenateş, E., Kahraman, R., Yüzbaşıoğlu, B., . . . Sökmen, H. M. (2011). Levofloxacin based sequential and triple therapy compared with standard plus probiotic combination for Helicobacter pylori eradication. Hepato-Gastroenterology, 58(109), 1148–1152. <a href="https://doi.org/10.5754/hge11075">https://doi.org/10.5754/hge11075</a></li>
<li class="show">Kale‐Pradhan, P. B., Mihaescu, A., & Wilhelm, S. M. (2015). Fluoroquinolone sequential Therapy for helicobacter pylori: A meta-analysis. Pharmacotherapy, 35(8), 719–730. <a href="https://doi.org/10.1002/phar.1614">https://doi.org/10.1002/phar.1614</a></li>
<li class="show">Lee, H., Hong, S. N., Min, B. H., Lee, J. H., Rhee, P. L., Lee, Y. C., & Kim, J. J. (2015). Comparison of efficacy and safety of Levofloxacin-containing versus standard sequential therapy in eradication of Helicobacter pylori infection in Korea. Digestive and Liver Disease, 47(2), 114–118. <a href="https://doi.org/10.1016/j.dld.2014.10.014">https://doi.org/10.1016/j.dld.2014.10.014</a></li>
<li class="show">Polat, Z., Kadayifci, A., Kantarcioglu, M., Ozcan, A., Emer, O., & Uygun, A. (2012). Comparison of levofloxacin-containing sequential and standard triple therapies for the eradication of Helicobacter pylori. European Journal of Internal Medicine, 23(2), 165–168. <a href="https://doi.org/10.1016/j.ejim.2011.02.011">https://doi.org/10.1016/j.ejim.2011.02.011</a></li>
<li class="show">Moghadaszadeh MFattahi E. (2012). Somi MH, Khaoshbaten M, Rosta T. The comparison between two treatment methods for H. pylori eradication with Two-week sequential regimens. J Arak Univer Med Sci., 15(61), 93–99.</li>
<li class="show">Seyyedmajidi, M., Falaknazi, K., Mirsattari, D., Zojaji, H., Roshani, M., Lahmi, F., . . . Zali, M. (2011). Correlation2 between creatinine clearance and Helicobacter pylori infection eradication with sequential and triple therapeutic regimens: A randomised clinical trial. Arab Journal of Gastroenterology, 12(3), 150–153. <a href="https://doi.org/10.1016/j.ajg.2011.07.004 ">https://doi.org/10.1016/j.ajg.2011.07.004 </a></li>
<li class="show">Eisig, J. N., Silva, F. M., Barbuti, R. C., Navarro-Rodriguez, T., Moraes-Filho, J. P., & Pedrazzoli, J., Jr. (2011). Helicobacter pylori antibiotic resistance in Brazil: Clarithromycin is still a good option. Arquivos de Gastroenterologia, 48(4), 261–264. <a href="https://doi.org/10.1590/S0004-28032011000400008">https://doi.org/10.1590/S0004-28032011000400008</a></li>
<li class="show">Moradniani, M., Mirbeik-Sabzevari, Z., Bahmani, M., Azadbakht, S., & Jaferian, S. (2017). Comparison of 7 - day concomitant therapy regimen versus classic triple therapy regimen in Helicobacter pylori eradication: A randomized clinical trial. Int J Pharm Sci Res, 8(8), 3568–3573.</li>
<li class="show">Moradniani, M., Azadbakht, S., & Jaferian, S. (2017). levofloxacin versus clarithromycin sequential therapy. Gastroenterology and Hepatology from Bed To Bench, •••, 10.</li>
</ol>
Downloads
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
