Methanol extract from Apium graveolens stimulate glucagon-like peptide-1 secretion (GLP-1), reduced dipeptidyl-peptidase-4 (DPP-4) and protect pancreatic b cells against glucotoxicity and H2O2 toxicity
DOI:
https://doi.org/10.15419/bmrat.v11i6.900Keywords:
Apium graveolens, anti-diabetic, glucagon-like peptide-1, insulin, dipeptidyl peptidase-4Abstract
Introduction: Glucagon-like peptide-1 (GLP-1) plays a critical role in glucose regulation by stimulating insulin secretion in a glucose-dependent manner. It enhances the functionality of pancreatic β-cells, minimizing apoptotic signals while promoting their proliferation and neogenesis. Consequently, leveraging plant-based GLP-1 agonists offers a promising approach to mitigating hyperglycemia in individuals with type 2 diabetes. This study aimed to explore the potential of methanol extract from Apium graveolens (commonly known as celery) to enhance insulin secretion, inhibit dipeptidyl peptidase-4 (DPP-4), and boost GLP-1 levels. Furthermore, it assessed celery's ability to protect pancreatic β cells against glucotoxicity and H2O2-induced toxicity in vitro. Additionally, we sought to identify the flavonoids present in the crude ethanolic extract of celery through HPLC/MS analysis.
Methods: We assessed cell viability, cell integrity (using lactate dehydrogenase (LDH) leakage assay), mitochondrial function (via ATP production), and insulin secretion in INS-1 pancreatic β-cells. GLP-1 activation and insulin sensitivity were evaluated in GLUTag cells, while DPP-4 activity was measured using an in vitro inhibitory assay.
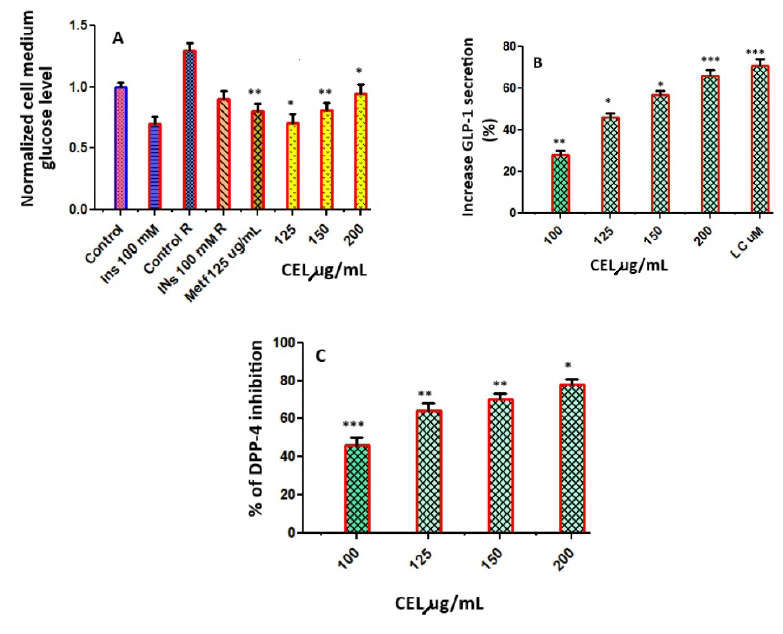
Results: HPLC/MS analysis revealed the presence of 11 phenolic compounds in celery. Treatment with varying concentrations of celery extract (100, 125, 150, 200 µg/mL) resulted in decreased cell death, significantly improved cell viability, and increased cellular ATP levels, thereby offering protection against glucotoxicity and H2O2-induced toxicity. Additionally, it boosted insulin production and reduced insulin resistance. The inhibitory effect of celery extract on DPP-4 activity, coupled with the increase in GLP-1 halflife, enhances insulin secretion from β cells, outlining celery's potential hypoglycemic mechanism.
Conclusion: The findings of this study underscore celery's therapeutic potential as a treatment option for individuals with type 2 diabetes, attributing to its ability to modulate insulin secretion, enhance pancreatic β-cell survival, and inhibit DPP-4, thereby increasing GLP-1 levels. This illuminates a promising avenue for further research and potential diabetes management strategies.
Published
Issue
Section
License
Copyright The Author(s) 2017. This article is published with open access by BioMedPress. This article is distributed under the terms of the Creative Commons Attribution License (CC-BY 4.0) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
